Abstract
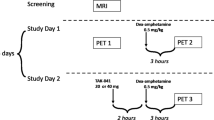
The radiotracer [11C]PHNO may have advantages over other dopamine (DA) D2/D3 receptor ligands because, as an agonist, it measures high-affinity, functionally active D2/D3 receptors, whereas the traditionally used radiotracer [11C]raclopride measures both high- and low-affinity receptors. Our aim was to take advantage of the strength of [11C]PHNO for measuring the small DA signal induced by nicotine, which has been difficult to measure in preclinical and clinical neuroimaging studies. Nicotine- and amphetamine-induced DA release in non-human primates was measured with [11C]PHNO and [11C]raclopride positron emission tomography (PET) imaging. Seven adult rhesus monkeys were imaged on a FOCUS 220 PET scanner after injection of a bolus of [11C]PHNO or [11C]raclopride in three conditions: baseline; preinjection of nicotine (0.1 mg/kg bolus+0.08 mg/kg infusion over 30 min); preinjection of amphetamine (0.4 mg/kg, 5 min before radiotracer injection). DA release was measured as change in binding potential (BPND). Nicotine significantly decreased BPND in the caudate (7±8%), the nucleus accumbens (10±7%), and in the globus pallidus (13±15%) measured with [11C]PHNO, but did not significantly decrease BPND in the putamen or the substantia nigra or in any region when measured with [11C]raclopride. Amphetamine significantly reduced BPND in all regions with both radiotracers. In the striatum, larger amphetamine-induced changes were detected with [11C]PHNO compared with [11C]raclopride (52–64% vs 33–35%, respectively). We confirmed that [11C]PHNO is more sensitive than [11C]raclopride to nicotine- and amphetamine-induced DA release. [11C]PHNO PET may be more sensitive to measuring tobacco smoking-induced DA release in human tobacco smokers.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barrett SP, Boileau I, Okker J, Pihl RO, Dagher A (2004). The hedonic response to cigarette smoking is proportional to dopamine release in the human striatum as measured by positron emission tomography and [11C]raclopride. Synapse 54: 65–71.
Brazell MP, Mitchell SN, Joseph MH, Gray JA (1990). Acute administration of nicotine increases the in vivo extracellular levels of dopamine, 3,4-dihydroxyphenylacetic acid and ascorbic acid preferentially in the nucleus accumbens of the rat: comparison with caudate–putamen. Neuropharmacology 29: 1177–1185.
Brody AL, London ED, Olmstead RE, Allen-Martinez Z, Shulenberger S, Costello MR et al (2010). Smoking-induced change in intrasynaptic dopamine concentration: effect of treatment for tobacco dependence. Psychiatry Res 183: 218–224.
Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL et al (2009a). Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology 34: 282–289.
Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S et al (2006). Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry 63: 808–816.
Brody AL, Olmstead RE, Abrams AL, Costello MR, Khan A, Kozman D et al (2009b). Effect of a history of major depressive disorder on smoking-induced dopamine release. Biol Psychiatry 66: 898–901.
Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P et al (2004). Smoking-induced ventral striatum dopamine release. Am J Psychiatry 161: 1211–1218.
Brown DJ, Luthra SK, Brady F, Prenant C, Dijkstra D, Wikstrom H (eds) (1997). Labelling of the D2-agonist-(+)-PHNO using [11C]-propionyl chloride. Proceedings of the XIIth International Symposium on Radiopharmaceutical Chemistry. John Wiley and Sons: Uppsala, Sweden.
CDC (2010). Vital signs: current cigarette smoking among adults aged >/=18 years—United States, 2005–2010. Morb Mortal Wkly Rep 60: 1207–1212.
Cumming P, Rosa-Neto P, Watanabe H, Smith D, Bender D, Clarke PB et al (2003). Effects of acute nicotine on hemodynamics and binding of [11C]raclopride to dopamine D2,3 receptors in pig brain. Neuroimage 19: 1127–1136.
Dewey SL, Brodie JD, Gerasimov M, Horan B, Gardner EL, Ashby CR Jr (1999). A pharmacologic strategy for the treatment of nicotine addiction. Synapse 31: 76–86.
Di Chiara G, Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278.
Domino EF, Tsukada H (2009). Nicotine sensitization of monkey striatal dopamine release. Eur J Pharmacol 607: 91–95.
Fuchs H, Nagel J, Hauber W (2005). Effects of physiological and pharmacological stimuli on dopamine release in the rat globus pallidus. Neurochem Int 47: 474–481.
Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T et al (2012). Affinity and selectivity of [(11) C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse 66: 489–500.
Gerasimov MR, Franceschi M, Volkow ND, Rice O, Schiffer WK, Dewey SL (2000). Synergistic interactions between nicotine and cocaine or methylphenidate depend on the dose of dopamine transporter inhibitor. Synapse 38: 432–437.
Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P et al (2006). Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem 97: 1089–1103.
Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S et al (2007a). Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab 27: 857–871.
Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S et al (2007b). Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab 27: 857–871.
Girgis RR, Xu X, Miyake N, Easwaramoorthy B, Gunn RN, Rabiner EA et al (2011). In vivo binding of antipsychotics to D(3) and D(2) receptors: a PET study in baboons with [(11)C]-(+)-PHNO. Neuropsychopharmacology 36: 887–895.
Gunn RN, Gunn SR, Turkheimer FE, Aston JAD, Cunningham VJ (2002). Positron emission tomography compartmental models: a basis pursuit strategy for kinetic modeling. J Cereb Blood Flow Metab 22: 1425–1439.
Hauber W, Fuchs H (2000). Dopamine release in the rat globus pallidus characterised by in vivo microdialysis. Behav Brain Res 111: 39–44.
Hwang DR, Kegeles LS, Laruelle M (2000). (−)-N-[(11)C]propyl-norapomorphine: a positron-labeled dopamine agonist for PET imaging of D(2) receptors. Nucl Med Biol 27: 533–539.
Ichise M, Ballinger JR, Golan H, Vines D, Luong A, Tsai S et al (1996). Noninvasive quantification of dopamine D2 receptors with iodine-123-IBF SPECT. J Nucl Med 37: 513–520.
Ichise M, Cohen RM, Carson RE (2008). Noninvasive estimation of normalized distribution volume: application to the muscarinic-2 ligand [(18)F]FP-TZTP. J Cereb Blood Flow Metab 28: 420–430.
Ichise M, Toyama H, Innis RB, Carson RE (2002). Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab 22: 1271–1281.
Imperato A, Mulas A, DiChiara G (1986). Nicotine preferentially stimulates dopamine release n the limbic system of freely moving rats. Eur J Pharmacol 132: 337–338.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN et al (2007). Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 27: 1533–1539.
Lammertsma AA, Hume SP (1996). Simplified reference tissue model for PET receptor studies. NeuroImage 4 (Part 1): 153–158.
Laruelle M (2000). Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab 20: 423–451.
Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL (1996). Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab 16: 834–840.
Logan J, Fowler JS, Volkow ND, Wolf A, Dewey SL, Schlyer D et al (1990). Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab 10: 740–747.
Marenco S, Carson RE, Berman KF, Herscovitch P, Weinberger DR (2004). Nicotine-induced dopamine release in primates measured with [11C]raclopride PET. Neuropsychopharmacology 29: 259–268.
Marshall DL, Redfern PH, Wonnacott S (1997). Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J Neurochem 68: 1511–1519.
Mizrahi R, Houle S, Vitcu I, Ng A, Wilson AA (2010). Side effects profile in humans of (11)C-(+)-PHNO, a dopamine D(2/3) agonist ligand for PET. J Nucl Med 51: 496–497.
Montgomery AJ, Lingford-Hughes AR, Egerton A, Nutt DJ, Grasby PM (2007). The effect of nicotine on striatal dopamine release in man: a [11C]raclopride PET study. Synapse 61: 637–645.
Morris E, Kim S, Sullivan J, Wang S, Normandin M, Constantinescu C et al (2013). Creating dynamic images of short-lived dopamine fluctuations with lp-ntPET: dopamine movies of cigarette smoking. JoVE. doi:10.3791/50358.
Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E et al (2006). Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse 60: 485–495.
Paxinos G, Huang X, Toga A (2000) The Rhesus Monkey Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA.
Sandiego CM, Weinzimmer D, Carson RE (2013). Optimization of PET-MR registrations for nonhuman primates using mutual information measures: a Multi-Transform Method (MTM). NeuroImage 64: 571–581.
Scott DJ, Domino EF, Heitzeg MM, Koeppe RA, Ni L, Guthrie S et al (2007). Smoking modulation of mu-opioid and dopamine D2 receptor-mediated neurotransmission in humans. Neuropsychopharmacology 32: 450–457.
Shim I, Javaid JI, Wirtshafter D, Jang SY, Shin KH, Lee HJ et al (2001). Nicotine-induced behavioral sensitization is associated with extracellular dopamine release and expression of c-Fos in the striatum and nucleus accumbens of the rat. Behav Brain Res 121: 137–147.
Shotbolt P, Tziortzi AC, Searle GE, Colasanti A, van der Aart J, Abanades S et al (2012). Within-subject comparison of [(11)C]-(+)-PHNO and [(11)C]raclopride sensitivity to acute amphetamine challenge in healthy humans. J Cereb Blood Flow Metab 32: 127–136.
Slifstein M, Kegeles LS, Xu X, Thompson JL, Urban N, Castrillon J et al (2010). Striatal and extrastriatal dopamine release measured with PET and [(18)F] fallypride. Synapse 64: 350–362.
Sullivan J, Kim S, Cosgrove K, Morris E (2013). Limitations of SRTM, logan graphical method, and equilibrium analysis for measuring transient dopamine release with [11C]raclopride PET. Am J Nucl Med Mol Imag 3: 247–260.
Takahashi H, Fujimura Y, Hayashi M, Takano H, Kato M, Okubo Y et al (2007). Enhanced dopamine release by nicotine in cigarette smokers: a double-blind, randomized, placebo-controlled pilot study. Int J Neuropsychopharmacol 11: 1–5.
Talhout R, Opperhuizen A, van Amsterdam JG (2007). Role of acetaldehyde in tobacco smoke addiction. Eur Neuropsychopharmacology 17: 627–636.
Tsukada H, Miyasato K, Kakiuchi T, Nishiyama S, Harada N, Domino EF (2002). Comparative effects of methamphetamine and nicotine on the striatal [(11)C]raclopride binding in unanesthetized monkeys. Synapse 45: 207–212.
Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M et al (2011). Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage 54: 264–277.
Villegier AS, Lotfipour S, McQuown SC, Belluzzi JD, Leslie FM (2007). Tranylcypromine enhancement of nicotine self-administration. Neuropharmacology 52: 1415–1425.
Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S et al (2008). First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: a [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology 33: 279–289.
Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P et al (2006). High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry 59: 389–394.
Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D et al (2005). Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9-ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem 48: 4153–4160.
Acknowledgements
The authors acknowledge the expertise of the staff of the Yale PET Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Gallezot, JD., Kloczynski, T., Weinzimmer, D. et al. Imaging Nicotine- and Amphetamine-Induced Dopamine Release in Rhesus Monkeys with [11C]PHNO vs [11C]raclopride PET. Neuropsychopharmacol 39, 866–874 (2014). https://doi.org/10.1038/npp.2013.286
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.286
Keywords
This article is cited by
-
Harmonization of [11C]raclopride brain PET images from the HR+ and HRRT: method development and validation in human subjects
EJNMMI Physics (2022)
-
To explore the mechanism of tobacco addiction using structural and functional MRI: a preliminary study of the role of the cerebellum-striatum circuit
Brain Imaging and Behavior (2022)
-
The influence of conditioned stimuli on [11C]-(+)-PHNO PET binding in tobacco smokers after a one week abstinence
Scientific Reports (2021)
-
Imaging synaptic dopamine availability in individuals at clinical high-risk for psychosis: a [11C]-(+)-PHNO PET with methylphenidate challenge study
Molecular Psychiatry (2021)
-
Extra-striatal D2/3 receptor availability in youth at risk for addiction
Neuropsychopharmacology (2020)