Abstract
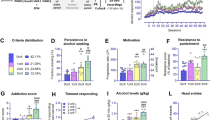
High rates of comorbidity between alcohol use disorder (AUD) and major depressive disorder (MDD) are reported. Preclinical models examining effects of primary depression on secondary AUD are currently absent, preventing adequate testing of drug treatment. Here, we combined social defeat-induced persistent stress (SDPS) and operant alcohol self-administration (SA) paradigms to assess causality between these two neuropsychiatric disorders. We then exploited guanfacine, an FDA-approved adrenergic agent reported to reduce drug craving in humans, against SDPS-induced modulation of operant alcohol SA. Wistar rats were socially defeated and isolated for a period of ⩾9 weeks, during which depression-like symptomatology (cognitive and social behavioral symptoms) was assessed. Subsequently, animals were subjected to a 5-month operant alcohol SA paradigm, examining acquisition, motivation, extinction, and cue-induced reinstatement of alcohol seeking. The effects of guanfacine on motivation and relapse were measured at >6 months following defeat. SDPS rats exhibited significant disruption of social and cognitive behavior, including short-term spatial and long-term social memory, several months following defeat. Notably, SDPS increased motivation to obtain alcohol, and cue-induced relapse vulnerability. Guanfacine reversed the SDPS-induced effects on motivation and relapse. Together, our model mimics core symptomatology of a sustained depressive-like state and a subsequent vulnerability to alcohol abuse. We show that SDPS is strongly associated with an enhanced motivation for alcohol intake and relapse. Finally, we show that the clinically employed drug guanfacine has potential as a novel treatment option in comorbid patients, as it effectively reduced the enhanced sensitivity to alcohol and alcohol-associated stimuli.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Akkerman S, Prickaerts J, Steinbusch H, Blokland A (2012). Object recognition testing: statistical considerations. Behav Brain Res 232: 317–322.
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders 4th edn. text rev. American Psychiatric Press: Washington, DC, USA.
Arnsten A, Jin L (2012). Guanfacine for the treatment of cognitive disorders: a century of discoveries at Yale. Yale J Biol Med 85: 45–58.
Arnsten AFT (2009). Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10: 410–422.
Artola A, von Frijtag J, Fermont P, Gispen W, Schrama L, Kamal A et al (2006). Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci 23: 261–272.
Austin M, Mitchell P, Goodwin G (2001). Cognitive deficits in depression: possible implications for functional neuropathology. Br J Psychiatry 178: 200–206.
Baune BT, Miller R, McAfoose J, Johnson M, Quirk F, Mitchell D (2010). The role of cognitive impairment in general functioning in major depression. Psychiatry Res 176: 183–189.
Bremner J, Narayan M, Anderson E, Staib L, Miller H, Charney D (2000). Hippocampal volume reduction in major depression. Am J Psychiatry 157: 115–118.
Briand L, Blendy J (2010). Molecular and genetic substrates linking stress and addiction. Brain Res 1314: 219–234.
Caldwell E, Riccio D (2010). Alcohol self-administration in rats: modulation by temporal parameters related to repeated mild social defeat stress. Alcohol 44: 265–274.
Davis L, Uezato A, Newell J, Frazier E (2008). Major depression and comorbid substance use disorders. Curr Opin Psychiatry 21: 14–18.
de Jong J, van der Vegt B, Buwalda B, Koolhaas J (2005). Social environment determines the long-term effects of social defeat. Physiol Behav 84: 87–95.
Der-Avakian A, Markou A (2012). The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci 35: 68–77.
Dere E, Huston J, De Souza Silva M (2007). The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev 31: 673–704.
Fanous S, Terwilliger E, Hammer RJ, Nikulina E (2011). Viral depletion of VTA BDNF in rats modulates social behavior, consequences of intermittent social defeat stress, and long-term weight regulation. Neurosci Lett 502: 192–196.
Femenía T, Gómez-Galán M, Lindskog M, Magara S (2012). Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Res 1476: 58–70.
Fone K, Porkess M (2008). Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev 32: 1087–1102.
Fox H, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan P et al (2012). Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol 26: 958–972.
Funk D, Harding S, Juzytsch W, Lê A (2005). Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 183: 341–349.
Gamo N, Arnsten A (2011). Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci 125: 282–296.
Goddard A, Ball S, Martinez J, Robinson M, Yang C, Russell J et al (2010). Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety 27: 339–350.
Golden S, Covington HI, Berton O, Russo S (2011). A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6: 1183–1191.
Goldstein R, Volkow N (2011). Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12: 652–669.
Hermes G, Li N, Duman C, Duman R (2011). Post-weaning chronic social isolation produces profound behavioral dysregulation with decreases in prefrontal cortex synaptic-associated protein expression in female rats. Physiol Behav 104: 354–359.
Ji X, Ji J, Zhang H, Li B (2008). Stimulation of alpha2-adrenoceptors suppresses excitatory synaptic transmission in the medial prefrontal cortex of rat. Neuropsychopharmacology 33: 2263–2271.
Kalivas P, Volkow N (2005). The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162: 1403–1413.
Kelly T, Daley D, Douaihy A (2012). Treatment of substance abusing patients with comorbid psychiatric disorders. Addict Behav 37: 11–24.
Koob G (2009). Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry 42: S32–S41.
Lê A, Funk D, Harding S, Juzytsch W, Fletcher P (2009). The role of noradrenaline and 5-hydroxytryptamine in yohimbine-induced increases in alcohol-seeking in rats. Psychopharmacology (Berl) 204: 477–488.
Lê A, Funk D, Juzytsch W, Coen K, Navarre B, Cifani C et al (2011). Effect of prazosin and guanfacine on stress-induced reinstatement of alcohol and food seeking in rats. Psychopharmacology (Berl) 218: 89–99.
Lee B, Tiefenbacher S, Platt D, Spealman R (2004). Pharmacological blockade of alpha2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology 29: 686–693.
Millan M, Agid Y, Brüne M, Bullmore E, Carter C, Clayton N et al (2012). Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 11: 141–168.
Nestler E, Carlezon WJ (2006). The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59: 1151–1159.
Nestler E, Hyman S (2012). Animal models of neuropsychiatric disorders. Nat Neurosci 13: 1161–1169.
Perry J, Joseph J, Jiang Y, Zimmerman R, Kelly T, Darna M et al (2011). Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev 65: 124–149.
Petrakis I, Gonzalez G, Rosenheck R, Krystal J (2002). Comorbidity of alcoholism and psychiatric disorders: an overview. Alcohol Res Health 26: 81–89.
Pettinati H (2004). Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry 56: 785–792.
Quan M, Tian Y, Xu K, Zhang T, Yang Z (2010). Post weaning social isolation influences spatial cognition, prefrontal cortical synaptic plasticity and hippocampal potassium ion channels in Wistar rats. Neuroscience 169: 214–222.
Reijmers L, Hoekstra K, Burbach J, van Ree J, Spruijt B (2001). Long-term impairment of social memory in the rat after social defeat is not restored by desglycinamide–vasopressin. Neurosci Lett 305: 145–148.
Schramm N, McDonald M, Limbird L (2001). The alpha(2a)-adrenergic receptor plays a protective role in mouse behavioral models of depression and anxiety. J Neurosci 21: 4875–4882.
Sinha R, Shaham Y, Heilig M (2011). Translational and reverse translational research on the role of stress in drug craving and relapse. Psychopharmacology (Berl) 218: 69–82.
Smith R, Aston-Jones G (2011). α(2) Adrenergic and imidazoline receptor agonists prevent cue-induced cocaine seeking. Biol Psychiatry 70: 712–719.
Sofuoglu M, Devito E, Waters A, Carroll K (2013). Cognitive enhancement as a treatment for drug addictions. Neuropharmacology 64: 452–463.
Van Bokhoven P, Oomen C, Hoogendijk W, Smit A, Lucassen P, Spijker S (2011). Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci 33: 1833–1840.
van Erp A, Miczek K (2001). Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav 73: 301–311.
Ventura R, Morrone C, Puglisi-Allegra S (2007). Prefrontal/accumbal catecholamine system determines motivational salience attribution to both reward- and aversion-related stimuli. Proc Natl Acad Sci USA 104: 5181–5186.
Von Frijtag J, Kamal A, Reijmers L, Schrama L, van den Bos R, Spruijt B (2001). Chronic imipramine treatment partially reverses the long-term changes of hippocampal synaptic plasticity in socially stressed rats. Neurosci Lett 309: 153–156.
Von Frijtag J, Reijmers L, Van der Harst J, Leus I, Van den Bos R, Spruijt B (2000). Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res 117: 137–146.
Wang M, Ramos BP, Paspalas CD, Shu YS, Simen A, Duque A et al (2007). alpha 2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 129: 397–410.
Wouda J, Riga D, De Vries W, Stegeman M, Van Mourik Y, Schetters D et al (2011). Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology 216: 267–277.
Acknowledgements
We thank Jon-Ruben van Rhijn and Dustin Schetters for their assistance in the behavioral experiments, as well as Michel van de Oever for his valuable advice on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Riga, D., Schmitz, L., van der Harst, J. et al. A Sustained Depressive State Promotes a Guanfacine Reversible Susceptibility to Alcohol Seeking in Rats. Neuropsychopharmacol 39, 1115–1124 (2014). https://doi.org/10.1038/npp.2013.311
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.311
Keywords
This article is cited by
-
Behavioral and accumbens synaptic plasticity induced by cues associated with restraint stress
Neuropsychopharmacology (2021)
-
Noradrenergic contributions to cue-driven risk-taking and impulsivity
Psychopharmacology (2021)
-
Temporal profiling of depression vulnerability in a preclinical model of sustained depression
Scientific Reports (2017)
-
Evaluation of Guanfacine as a Potential Medication for Alcohol Use Disorder in Long-Term Drinking Rats: Behavioral and Electrophysiological Findings
Neuropsychopharmacology (2015)