Abstract
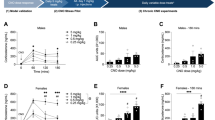
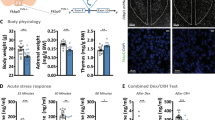
The corticotropin-releasing factor (CRF) neuropeptide is found to have a pivotal role in the regulation of the behavioral and neuroendocrine responses to stressful challenges. Here, we studied the involvement of the hypothalamic CRF in learning under stressful conditions. We have used a site-specific viral approach to knockdown (KD) CRF expression in the paraventricular nucleus of the hypothalamus (PVN). The two-way shuttle avoidance (TWSA) task was chosen to assess learning and memory under stressful conditions. Control animals learned to shuttle from one side to the other to avoid electrical foot shock by responding to a tone. Novel object and social recognition tasks were used to assess memory under less stressful conditions. KD of PVN-CRF expression decreased the number of avoidance responses in a TWSA session under moderate (0.8 mA), but not strong (1.5 mA), stimulus intensity compared to control rats. On the other hand, KD of PVN-CRF had no effect on memory performance in the less stressful novel object or social recognition tasks. Interestingly, basal or stress-induced corticosterone levels in CRF KD rats were not significantly different from controls. Taken together, the data suggest that the observed impairment was not a result of alteration in HPA axis activity, but rather due to reduced PVN-CRF activity on other brain areas. We propose that hypothalamic CRF is centrally involved in learning under moderate stressful challenge. Under ‘basal’ (less stressful) conditions or when the intensity of the stress is more demanding, central CRF ceases to be the determinant factor, as was indicated by performances in the TWSA with higher stimulus intensity or in the less stressful tasks of object and social recognition.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Aguilera G, Nikodemova M, Wynn PC, Catt KJ (2004). Corticotropin releasing hormone receptors: two decades later. Peptides 25: 319–329.
Antoni FA (1993). Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol 14: 76–122.
Bangasser DA, Shors TJ (2010). Critical brain circuits at the intersection between stress and learning. Neurosci Biobehav Rev 34: 1223–1233.
Bergado JA, Lucas M, Richter-Levin G (2011). Emotional tagging—a simple hypothesis in a complex reality. Prog Neurobiol 94: 64–76.
Choi JS, Cain CK, LeDoux JE (2010). The role of amygdala nuclei in the expression of auditory signaled two-way active avoidance in rats. Learn Mem 17: 139–147.
Elliott E, Ezra-Nevo G, Regev L, Neufeld-Cohen A, Chen A (2010). Resilience to social stress coincides with functional DNA methylation of the CRF gene in adult mice. Nat Neurosci 13: 1351–1353.
Fluttert M, Dalm S, Oitzl MS (2000). A refined method for sequential blood sampling by tail incision in rats. Lab Anim 34: 372–378.
Heinrichs SC (2003). Modulation of social learning in rats by brain corticotropin-releasing factor. Brain Res 994: 107–114.
Hubbard DT, Nakashima BR, Lee I, Takahashi LK (2007). Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience 150: 818–828.
Joels M, Baram TZ (2009). The neuro-symphony of stress. Nat Rev Neurosci 10: 459–466.
Kant GJ, Mougey EH, Pennington LL, Meyerhoff JL (1983). Graded footshock stress elevates pituitary cyclic AMP and plasma beta-endorphin, beta-LPH corticosterone and prolactin. Life Sci 33: 2657–2663.
Kasahara M, Groenink L, Kas MJ, Bijlsma EY, Olivier B, Sarnyai Z (2011). Influence of transgenic corticotropin-releasing factor (CRF) over-expression on social recognition memory in mice. Behav Brain Res 218: 357–362.
Kehne JH, Cain CK (2010). Therapeutic utility of non-peptidic CRF1 receptor antagonists in anxiety, depression, and stress-related disorders: evidence from animal models. Pharmacol Ther 128: 460–487.
Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flugge G, Korte SM et al (2011). Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev 35: 1291–1301.
Lee EH, Lee CP, Wang HI, Lin WR (1993). Hippocampal CRF, NE, and NMDA system interactions in memory processing in the rat. Synapse 14: 144–153.
Lloyd RB, Nemeroff CB (2011). The role of corticotropin-releasing hormone in the pathophysiology of depression: therapeutic implications. Curr Top Med Chem 11: 609–617.
McGaugh JL (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28.
Merino JJ, Cordero MI, Sandi C (2000). Regulation of hippocampal cell adhesion molecules NCAM and L1 by contextual fear conditioning is dependent upon time and stressor intensity. Eur J Neurosci 12: 3283–3290.
Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P et al (2003). Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci 6: 1100–1107.
Natelson BH, Creighton D, McCarty R, Tapp WN, Pitman D, Ottenweller JE (1987). Adrenal hormonal indices of stress in laboratory rats. Physiol Behav 39: 117–125.
Natelson BH, Tapp WN, Adamus JE, Mittler JC, Levin BE (1981). Humoral indices of stress in rats. Physiol Behav 26: 1049–1054.
Oades RD, Rivet JM, Taghzouti K, Kharouby M, Simon H, Le Moal M (1987). Catecholamines and conditioned blocking: effects of ventral tegmental, septal and frontal 6-hydroxydopamine lesions in rats. Brain Res 406: 136–146.
Pacak K, Palkovits M, Kopin IJ, Goldstein DS (1995). Stress-induced norepinephrine release in the hypothalamic paraventricular nucleus and pituitary-adrenocortical and sympathoadrenal activity: in vivo microdialysis studies. Front Neuroendocrinol 16: 89–150.
Paxinos G, Watson C (1997) The Rat Brain in Stereotaxic Coordinates 3rd edn. Academic Press: New York.
Pezze MA, Feldon J (2004). Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol 74: 301–320.
Quirarte GL, Galvez R, Roozendaal B, McGaugh JL (1998). Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res 808: 134–140.
Quiroz C, Martinez I, Quirarte GL, Morales T, Diaz-Cintra S, Prado-Alcala RA (2003). Enhanced inhibitory avoidance learning prevents the memory-impairing effects of post-training hippocampal inactivation. Exp Brain Res 153: 400–402.
Rabasa C, Munoz-Abellan C, Daviu N, Nadal R, Armario A (2011). Repeated exposure to immobilization or two different footshock intensities reveals differential adaptation of the hypothalamic-pituitary-adrenal axis. Physiol Behav 103: 125–133.
Radwanska K, Nikolaev E, Kaczmarek L (2010). Central noradrenergic lesion induced by DSP-4 impairs the acquisition of avoidance reactions and prevents molecular changes in the amygdala. Neurobiol Learn Mem 94: 303–311.
Regev L, Tsoory M, Gil S, Chen A (2012). Site-specific genetic manipulation of amygdala corticotropin-releasing factor reveals its imperative role in mediating behavioral response to challenge. Biol Psychiatry 71: 317–326.
Reyes BA, Valentino RJ, Xu G, Van Bockstaele EJ (2005). Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci 22: 93–106.
Rodaros D, Caruana DA, Amir S, Stewart J (2007). Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience 150: 8–13.
Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ (2002). Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc Natl Acad Sci USA 99: 13908–13913.
Row BW, Dohanich GP (2008). Post-training administration of corticotropin-releasing hormone (CRH) enhances retention of a spatial memory through a noradrenergic mechanism in male rats. Neurobiol Learn Mem 89: 370–378.
Straube T, Korz V, Frey JU (2003). Bidirectional modulation of long-term potentiation by novelty-exploration in rat dentate gyrus. Neurosci Lett 344: 5–8.
Swanson LW, Sawchenko PE, Rivier J, Vale WW (1983). Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36: 165–186.
Terranova ML, Cirulli F, Laviola G (1999). Behavioral and hormonal effects of partner familiarity in periadolescent rat pairs upon novelty exposure. Psychoneuroendocrinology 24: 639–656.
Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A (2003). Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron 39: 401–407.
Valentino RJ, Van Bockstaele E (2008). Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol 583: 194–203.
Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB (2005). Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci 25: 5389–5396.
Acknowledgements
This research was funded by a grant from the Institute for the Study of Affective Neuroscience University of Haifa, which was endowed by the Hope for Depression Research Foundation. We thank Dr Rachel Anunu for her outstanding help with corticosterone quantification.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lucas, M., Chen, A. & Richter-Levin, G. Hypothalamic Corticotropin-Releasing Factor is Centrally Involved in Learning Under Moderate Stress. Neuropsychopharmacol 38, 1825–1832 (2013). https://doi.org/10.1038/npp.2013.82
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.82