Abstract
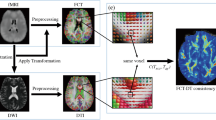
Post-mortem studies have demonstrated alterations in superficial white matter (SWM) in schizophrenia patients. Diffusion tensor imaging (DTI) can be used to assess SWM in vivo, and compare SWM fractional anisotropy (FA) in schizophrenia patients vs healthy controls. The assessment of SWM in vivo also provides an opportunity to identify novel neural correlates of cognitive performance, and potential cognitive impairment in schizophrenia patients. Forty-four patients with schizophrenia and 44 matched healthy controls underwent neuroimaging and cognitive protocols. Using an SWM mask and tract-based spatial statistics, differences in SWM-FA were examined between groups. SWM-FA clusters different between groups were then used to predict cognitive performance with multiple linear regression. The relative contribution of SWM fiber subtypes (deep white matter extensions vs U-fibers and intraregional fibers) from significantly different clusters was examined. Compared to controls, patients with schizophrenia had reduced FA in five SWM clusters: the largest a left posterior parieto-occipital cluster, followed by four clusters in the left frontal lobe. SWM-FA in the frontal lobe clusters predicted attention, working memory, and processing speed performance in healthy controls, but not in patients with schizophrenia. The majority of streamlines tracked from these clusters were restricted to U-fibers and intraregional fibers, rather than deep white matter extensions. Our analyses revealed prominent SWM disruption in patients with schizophrenia compared to controls. SWM–cognition relationships shown in healthy individuals were disrupted in patients with schizophrenia. SWM may be an important neurobiological substrate of cognitive performance and a novel cortical treatment target for cognitive deficits in schizophrenia patients.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Akbarian S, Bunney WE Jr., Potkin SG, Wigal SB, Hagman JO, Sandman CA et al (1993). Altered distribution of nicotinamide-adenine dinucleotide phosphate-diaphorase cells in frontal lobe of schizophrenics implies disturbances of cortical development. Arch Gen Psychiatry 50: 169–177.
Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE Jr., Jones EG (1996). Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry 53: 425–436.
Avants BB, Epstein CL, Grossman M, Gee JC (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12: 26–41.
Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A (2008). Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci 28: 9239–9248.
Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S et al (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50: 1077–1088.
Buckner RL, Andrews-Hanna JR, Schacter DL (2008). The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38.
Catani M, Dell'acqua F, Vergani F, Malik F, Hodge H, Roy P et al (2012). Short frontal lobe connections of the human brain. Cortex 48: 273–291.
Chun JJ, Shatz CJ (1989). Interstitial cells of the adult neocortical white matter are the remnant of the early generated subplate neuron population. J Comp Neurol 282: 555–569.
Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR et al (2003). White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry 60: 443.
Defelipe J, Fields RD, Hof PR, Hoistad M, Kostovic I, Meyer G et al (2010). Cortical white matter: beyond the pale remarks, main conclusions and discussion. Front Neuroanat 4: 4.
Dickinson D, Ramsey ME, Gold JM (2007). Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry 64: 532.
Dreher JC, Koch P, Kohn P, Apud J, Weinberger DR, Berman KF (2012). Common and differential pathophysiological features accompany comparable cognitive impairments in medication-free patients with schizophrenia and in healthy aging subjects. Biol Psychiatry 71: 890–897.
Eastwood S, Harrison P (2005). Interstitial white matter neuron density in the dorsolateral prefrontal cortex and parahippocampal gyrus in schizophrenia. Schizophr Res 79: 181–188.
Ehrlich S, Brauns S, Yendiki A, Ho BC, Calhoun V, Schulz SC et al (2012). Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophr Bull 38: 1050–1062.
Eisenberg DP, Berman KF (2010). Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology 35: 258–277.
Fioravanti M, Carlone O, Vitale B, Cinti ME, Clare L (2005). A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol Rev 15: 73–95.
First MB Sr, Gibbon M, Williams JBW (1995) Strucutured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. Biometrics Research: New York, NY, USA.
Fornito A, Zalesky A, Pantelis C, Bullmore ET (2012). Schizophrenia, neuroimaging and connectomics. NeuroImage 62: 2296–2314.
Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102: 9673.
Fransson P, Marrelec G (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage 42: 1178–1184.
Gerretsen P, Chakravarty MM, Mamo D, Menon M, Pollock BG, Rajji TK et al (2012). Frontotemporoparietal asymmetry and lack of illness awareness in schizophrenia. Hum Brain Mapp 34: 1035–1043.
Greicius MD, Krasnow B, Reiss AL, Menon V (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 100: 253–258.
Jones DK (2004). The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med 51: 807–815.
Joshi D, Fung SJ, Rothwell A, Weickert CS (2012). Higher gamma-aminobutyric acid neuron density in the white matter of orbital frontal cortex in schizophrenia. Biol Psychiatry 72: 725–733.
Kay SR, Fiszbein A, Opler LA (1987). The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 13: 261–276.
McIntosh AM, Maniega SM, Lymer GKS, McKirdy J, Hall J, Sussmann JED et al (2008). White matter tractography in bipolar disorder and schizophrenia. Biol Psychiatry 64: 1088–1092.
Menon V, Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214: 655–667.
Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR et al (2005). Regionally specific disturbance of dorsolateral prefrontal–-hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry 62: 379–386.
Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH et al (1992). Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 41: 237–248.
Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC (2009). Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry 66: 811–822.
Mori S, Wakana S, Van Zijl PCM, Nagae-Poetscher L (2005) MRI atlas of human white matter. Elsevier Science: London.
Nakamura M, Nestor PG, Levitt JJ, Cohen AS, Kawashima T, Shenton ME et al (2008). Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain 131 (Part 1): 180–195.
Oertel-Knochel V, Linden DE (2011). Cerebral asymmetry in schizophrenia. Neuroscientist 17: 456–467.
Oishi K, Zilles K, Amunts K, Faria A, Jiang H, Li X et al (2008). Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. NeuroImage 43: 447–457.
Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I et al (2010). White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry 167: 451–458.
Phillips OR, Nuechterlein KH, Asarnow RF, Clark KA, Cabeen R, Yang Y et al (2011). Mapping corticocortical structural integrity in schizophrenia and effects of genetic liability. Biol Psychiatry 70: 680–689.
Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL (2001). A default mode of brain function. Proc Natl Acad Sci USA 98: 676–682.
Rajji TK, Ismail Z, Mulsant BH (2009). Age at onset and cognition in schizophrenia: meta-analysis. Br J Psychiatry 195: 286–293.
Reichenberg AA (2010). The assessment of neuropsychological functioning in schizophrenia. Dialog Clin Neurosci 12: 383.
Smith SM (2002). Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155.
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE et al (2006). Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31: 1487–1505.
Smith SM, Nichols TE (2009). Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44: 83–98.
Tanskanen P, Ridler K, Murray GK, Haapea M, Veijola JM, Jaaskelainen E et al (2010). Morphometric brain abnormalities in schizophrenia in a population-based sample: relationship to duration of illness. Schizophr Bull 36: 766–777.
Voineskos AN, Felsky D, Kovacevic N, Tiwari AK, Zai C, Chakravarty MM et al (2012). Oligodendrocyte genes, white matter tract integrity, and cognition in schizophrenia. Cereb Cortex e-pub ahead of print 6 July 2012 (doi:10.1093/cercor/bhs188).
Voineskos AN, Lobaugh NJ, Bouix S, Rajji TK, Miranda D, Kennedy JL et al (2010). Diffusion tensor tractography findings in schizophrenia across the adult lifespan. Brain 133 (Part 5): 1494–1504.
Wechsler D (2001) Wechsler Test of Adult Reading. Harcourt Assessment: San Antonio, TX.
Whitfield-Gabrieli S, Ford JM (2012). Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8: 49–76.
Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW et al (2009). Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 106: 1279–1284.
Yang Y, Fung SJ, Rothwell A, Tianmei S, Weickert CS (2011). Increased interstitial white matter neuron density in the dorsolateral prefrontal cortex of people with schizophrenia. Biol Psychiatry 69: 63–70.
Yu Q, Sui J, Rachakonda S, He H, Pearlson G, Calhoun VD (2011). Altered small-world brain networks in temporal lobe in patients with schizophrenia performing an auditory oddball task. Front Syst Neurosci 5: 7.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research, Ontario Mental Health Foundation, the Brain and Behavior Research Foundation (formerly known as NARSAD), the Centre for Addiction and Mental Health (CAMH), and the CAMH Foundation through the Kimel Family, Koerner New Scientist Award, and Paul E Garfinkel New Investigator Catalyst Award. No sponsor or funder played any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Dr Mulsant currently receives research support from Bristol-Myers Squibb (medications for a NIH-funded clinical trial) and Pfizer (medications for a NIH-funded clinical trial). He directly owns stocks of General Electric (<$5000). Within the past 3 years, he has also received some travel support from Roche.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Nazeri, A., Chakravarty, M., Felsky, D. et al. Alterations of Superficial White Matter in Schizophrenia and Relationship to Cognitive Performance. Neuropsychopharmacol 38, 1954–1962 (2013). https://doi.org/10.1038/npp.2013.93
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2013.93
Keywords
This article is cited by
-
Psychosis spectrum illnesses as disorders of prefrontal critical period plasticity
Neuropsychopharmacology (2023)
-
U-fiber diffusion kurtosis and susceptibility characteristics in relapsing–remitting multiple sclerosis may be related to cognitive deficits and neurodegeneration
European Radiology (2023)
-
Interactive effects of polygenic risk and cognitive subtype on brain morphology in schizophrenia spectrum and bipolar disorders
European Archives of Psychiatry and Clinical Neuroscience (2022)
-
Altered microstructural properties of superficial white matter in patients with Parkinson’s disease
Brain Imaging and Behavior (2022)
-
The origin and development of subcortical U-fibers in gyrencephalic ferrets
Molecular Brain (2020)