Abstract
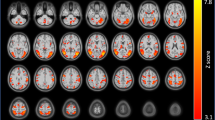
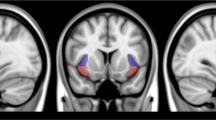
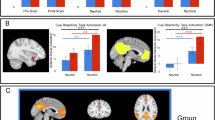
The insula plays a critical role in maintaining nicotine dependence and reactivity to smoking cues. More broadly, the insula and the dorsal anterior cingulate cortex (dACC) are key nodes of the salience network (SN), which integrates internal and extrapersonal information to guide behavior. Thus, insula–dACC interactions may be integral in processing salient information such as smoking cues that facilitate continued nicotine use. We evaluated functional magnetic resonance imaging (fMRI) data from nicotine-dependent participants during rest, and again when they viewed smoking-related images. Greater insula–dACC coupling at rest was significantly correlated with enhanced smoking cue-reactivity in brain areas associated with attention and motor preparation, including the visual cortex, right ventral lateral prefrontal cortex, and the dorsal striatum. In an independent cohort, we found that insula–dACC connectivity was stable over 1-h delay and was not influenced by changes in subjective craving or expired carbon monoxide, suggesting that connectivity strength between these regions may be a trait associated with heightened cue-reactivity. Finally, we also showed that insula reactivity to smoking cues correlates with a rise in cue-reactivity throughout the entire SN, indicating that the insula’s role in smoking cue-reactivity is not functionally independent, and may actually represent the engagement of the entire SN. Collectively, these data provide a more network-level understanding of the insula’s role in nicotine dependence and shows a relationship between inherent brain organization and smoking cue-reactivity.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM et al (2010). The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct 214: 5–6.
Andrews-Hanna JR, Snyder AZ, Vincent JL, Lustig C, Head D, Raichle ME et al (2007). Disruption of large-scale brain systems in advanced aging. Neuron 56: 924–935.
Aron AR, Robbins TW, Poldrack RA (2004). Inhibition and the right inferior frontal cortex. Trends Cogn Sci 8: 170–177.
Buckner RL, Carroll DC (2007). Self-projection and the brain. Trends Cogn Sci 11: 49–57.
Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF (2009). The role of nicotine in smoking: a dual-reinforcement model. Nebr Symp Motiv 55: 91–109.
Corbetta M, Patel G, Shulman GL (2008). The reorienting system of the human brain: from environment to theory of mind. Neuron 58: 306–324.
Corbetta M, Shulman GL (2002). Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci 3: 215–229.
Cox LS, Tiffany ST, Christen AG (2001). Evaluation of the brief questionnaire of smoking urges (QSU—brief) in laboratory and clinical settings. Nicotine Tob Res 3: 7–16.
De Ruiter MB, Veltman DJ, Goudriaan AE, Oosteriaan J, Sjoerds Z, van den Brink W (2009). Response perseveration and ventral prefrontal sensitivity to reward and punishment in male problem gamblers and smokers. Neuropsychopharmacology 34: 1027–1038.
Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA et al (2007). Distinct brain networks for adaptive and stable task control in humans. PNAS 104: 11073–11078.
Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC et al (2006). A core system of the implementation of task sets. Neuron 50: 799–812.
Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y et al (2012). Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage 60: 252–262.
Fagerström KO (1978). Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 3: 235–241.
Ferguson SG, Shiffman S (2009). The relevance and treatment of cue- induced cravings in tobacco dependence. J Subst Abuse Treat 36: 235–243.
Forget B, Pushparaj A, Le Foll B (2010). Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry 68: 265–271.
Gusnard DA, Akbudak E, Shulman GL, Raichle ME (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. PNAS 98: 4259–4262.
Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM (2010). The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage 50: 1313–1319.
Hanlon CA, Dowdle LT, Naselaris T, Canterberry M, Cortes BM (2014). Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depend 143: 206–212.
Haruno M, Kawato M (2005). Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol 95: 948–959.
Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM (2006). Medial prefrontal activity differentiates self from close others. Soc Cogn Affect Neurosci 1: 18–25.
Ito R, Dalley JW, Robbins TW, Everitt BJ (2002). Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J Neurosci 22: 6247–6253.
Janes AC, Farmer S, Frederick BB, Nickerson LD, Lukas SE (2014a). An increase in tobacco craving is associated with enhanced medial prefrontal cortex network coupling. PloS One 9: e88228.
Janes AC, Jensen JE, Farmer SL, Frederick BB, Pizzagalli DA, Lukas SE (2013a). GABA levels in the dorsal anterior cingulate cortex associated with difficulty ignoring smoking-related cues in tobacco-dependent volunteers. Neuropsychopharmacology 38: 1113–1120.
Janes AC, Nickerson LD, Frederick BB, Kaufman MJ (2012). Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend 125: 252–259.
Janes AC, Park MT, Farmer S, Chakravarty MM (2014b). Striatal morphology is associated with tobacco cigarette craving. Neuropsychopharmacology 40: 406–411.
Janes AC, Pizzagalli DA, Richardt S, Frederick BB, Chuzi S, Pachas G et al (2010). Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry 67: 722–729.
Janes AC, Ross RR, Farmer S, Frederick BB, Nickerson LD, Lukas SE et al (2013b). Memory retrieval of smoking-related images induce greater insula activation as revealed by an fMRI-based delayed matching to sample task. Addict Biol; e-pub ahead of print 22 November 2013; doi: 10.1111/adb.12112.
Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS et al (2010). Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J Neurosci Methods 189: 233–245.
Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelly WM (2004). Medial prefrontal activity predicts memory for self. Cereb Cortex 14: 647–654.
Menon V (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15: 483–506.
Menon V, Uddin LQ (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214: 655–667.
Miller EM, Shankar MU, Knutson B, McClure SM (2014). Dissociating motivation from reward in human striatal activity. J Cogn Neurosci 26: 1075–1084.
Moran JM, Heatherton TF, Kelley WM (2006). Modulation of cortical midline structures by implicit and explicit self- relevance evaluation. Soc Neurosci 4: 197–211.
Naqvi NH, Bechara A (2009). The hidden island of addiction: the insula. Trends Neurosci 32: 56–67.
Naqvi NH, Gaznick N, Tranel D, Bechara A (2014). The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann NY Acad Sci 1316: 53–70.
Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007). Damage to the insula disrupts addiction to cigarette smoking. Science 315: 531–534.
Nelson SM, Dosenbach NU, Cohen AL, Wheeler ME, Schlagger BL, Petersen SE (2010). Role of the anterior insula in task-level control and focal attention. Brain Struct Funct 214: 669–680.
Oppenheim AV, Schafer RW (1975) Digital Signal Processing. Prentice-Hall International: London, 1975 pp 242.
Ploran EJ, Nelson SM, Velanova K, Donaldson DI, Petersen SE, Wheeler ME (2007). Evidence accumulation and the moment of recognition: dissociating perceptual recognition processes using fMRI. J Neurosci 27: 11912–11924.
Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA (2004). Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci 24: 3554–3562.
Pratte MS, Ling S, Swisher JD, Tong F (2013). How attention extracts objects from noise. J Neurophysiol 110: 1346–1356.
Pushparaj A, Hamani C, Yu W, Shin DS, Kang B, Nobrega JN et al (2013). Electrical stimulation of the insula regions attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology 38: 690–698.
See RE, Elliott JC, Feltenstein MW (2007). The role of dorsal vs ventral striatal pathways in cocaine-seeking behavior after prolonged abstinence in rats. Psychopharmacology 194: 321–331.
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27: 2349–2356.
Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA (2013). Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Bio Psychiatry 74: 538–546.
Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Stein EA (2013b). Insula’s functional connectivity with ventromedial prefrontal cortex mediates the impact of trait alexithymia on state tobacco craving. Psychopharmacology 228: 143–155.
Verbruggen F, Logan GD (2009). Automaticity of cognitive control: goal priming in response-inhibition paradigms. J Exp Psychol Learn Mem Cogn 35: 1381–1388.
Volkow ND, Wang G, Telang F, Fowler JS, Logan J, Childress AR et al (2006). Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26: 6583–6588.
Wager TD, Barret LF (2004). From affect to control: functional specialization of the insula, motivation and regulation. Available at PsychExtra http://www.columbia.edu/cu/psychology/tor/ accessed on 20 November 2012.
Yamada H, Matsumoto N, Kimura M (2004). Tonically active neurons in the primate caudate nucleus and putamen differentially encode instructed motivational outcomes of action. J Neurosci 24: 3500–3520.
Yokum S, Ng J, Stice E (2011). Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity 19: 1775–1783.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
PowerPoint slides
Rights and permissions
About this article
Cite this article
Janes, A., Farmer, S., Peechatka, A. et al. Insula–Dorsal Anterior Cingulate Cortex Coupling is Associated with Enhanced Brain Reactivity to Smoking Cues. Neuropsychopharmacol 40, 1561–1568 (2015). https://doi.org/10.1038/npp.2015.9
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2015.9
This article is cited by
-
Data-driven connectivity profiles relate to smoking cessation outcomes
Neuropsychopharmacology (2024)
-
Multimodal 7T imaging reveals enhanced functional coupling between salience and frontoparietal networks in young adult tobacco cigarette smokers
Brain Imaging and Behavior (2024)
-
Effect of progesterone administration in male and female smokers on nicotine withdrawal and neural response to smoking cues: role of progesterone conversion to allopregnanolone
Biology of Sex Differences (2022)
-
Deep rTMS of the insula and prefrontal cortex in smokers with schizophrenia: Proof-of-concept study
Schizophrenia (2022)
-
Imaging suicidal thoughts and behaviors: a comprehensive review of 2 decades of neuroimaging studies
Molecular Psychiatry (2020)