Abstract
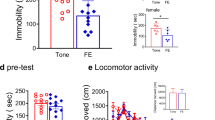
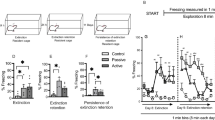
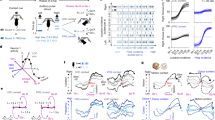
Current treatments for stress-related psychiatric disorders, such as depression and posttraumatic stress disorder (PTSD), are inadequate. Cognitive behavioral psychotherapies, including exposure therapy, are an alternative to pharmacotherapy, but the neurobiological mechanisms are unknown. Preclinical models demonstrating therapeutic effects of behavioral interventions are required to investigate such mechanisms. Exposure therapy bears similarity to extinction learning. Thus, we investigated the therapeutic effects of extinction learning as a behavioral intervention to model exposure therapy in rats, testing its effectiveness in reversing chronic stress-induced deficits in cognitive flexibility and coping behavior that resemble dimensions of depression and PTSD. Rats were fear-conditioned by pairing a tone with footshock, and then exposed to chronic unpredictable stress (CUS) that induces deficits in cognitive set-shifting and active coping behavior. They then received an extinction learning session as a therapeutic intervention by repeated exposure to the tone with no shock. Effects on cognitive flexibility and coping behavior were assessed 24 h later on the attentional set-shifting test or shock-probe defensive burying test, respectively. Extinction reversed the CUS-induced deficits in cognitive flexibility and coping behavior, and increased phosphorylation of ribosomal protein S6 in the medial prefrontal cortex (mPFC) of stress-compromised rats, suggesting a role for activity-dependent protein synthesis in the therapeutic effect. Inhibiting protein synthesis by microinjecting anisomycin into mPFC blocked the therapeutic effect of extinction on cognitive flexibility. These results demonstrate the utility of extinction as a model by which to study mechanisms underlying exposure therapy, and suggest these mechanisms involve protein synthesis in the mPFC, the further study of which may identify novel therapeutic targets.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Beck AT (1976) Cognitive Therapy and the Emotional Disorders. Int. Univ. Press: New York.
Beck AT (2005). The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry 62: 953–959.
Biehn TL, Contractor A, Elhai JD, Tamburrino M, Fine TH, Prescott MR et al (2013). Relations between the underlying dimensions of PTSD and major depression using an epidemiological survey of deployed Ohio National Guard soldiers. J Affective Disord 144: 106–111.
Biever A, Valjent E, Puighermanal E (2015). Ribosomal protein S6 phosphorylation in the nervous system: From regulation to function. Front Mol Neurosci 8: 75.
Birrell JM, Brown VJ (2000). Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 20: 4320–4324.
Bondi CO, Barrera G, Lapiz MDS, Bédard T, Mahan A, Morilak DA. (2007). Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuropsychopharmacol Biol Psychiatry 31: 482–495.
Bondi CO, Jett JD, Morilak DA (2010). Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 34: 913–923.
Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA (2008). Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33: 320–331.
Brewin CR (1996). Theoretical foundations of cognitive-behavior therapy for anxiety and depression. Annu Rev Psychol 47: 33–57.
Bryant RA, Kenny L, Joscelyne A, Rawson N, Maccallum F, Cahill C et al (2014). Treating prolonged grief disorder: a randomized clinical trial. JAMA Psychiatry 71: 1332–1339.
Buffington SA, Huang W, Costa-Mattioli M (2014). Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci 37: 17–38.
Coles ME, Heimberg RG (2002). Memory biases in the anxiety disorders: current status. Clin Psychol Rev 22: 587–627.
de Kleine RA, Rothbaum BO, van Minnen A (2013). Pharmacological enhancement of exposure-based treatment in PTSD: a qualitative review. Eur J Psychotraumatol 4: 21626.
Donegan JJ, Patton MS, Chavera TA, Berg KA, Morilak DA, Girotti M et al (2015). Interleukin-6 attenuates serotonin2A receptor signaling by activating the JAK-STAT pathway. Mol Pharmacol 87: 492–500.
Drevets WC (2000). Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 126: 413–431.
Duman RS, Li N, Liu R-J, Duric V, Aghajanian G (2012). Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology 62: 35–41.
Elhai JD, Carvalho LF, Miguel FK, Palmieri PA, Primi R, Christopher Frueh B (2011). Testing whether posttraumatic stress disorder and major depressive disorder are similar or unique constructs. J Anxiety Disord 25: 404–410.
Elzinga BM, Bremner JD (2002). Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J Affect Disord 70: 1–17.
Foa EB, Meadows EA (1997). Psychosocial treatments for posttraumatic stress disorder: a critical review. Ann Rev Psychol 48: 449–480.
Green MK, Rani CSS, Joshi A, Soto-Piña AE, Martinez PA, Frazer A et al (2011). Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience 192: 438–451.
Hayes AM, Beevers CG, Feldman GC, Laurenceau JP, Perlman C (2005). Avoidance and processing as predictors of symptom change and positive growth in an integrative therapy for depression. Int J Behav Med 12: 111–122.
Hoffmann SG (2008). Cognitive processes during fear acquisition and extinction in animals and humans: implications for exposure therapy of anxiety disorders. Clin Psychol Rev 28: 199–210.
Hollon SD, Thase ME, Markowitz JC (2002). Treatment and prevention of depression. Psychol Sci Public Interest 3: 39–77.
Janicak PG, Davis JM, Preskorn SH, Ayd FJ . Treatment with antidepressants. In: Janicak PG, Davis JM, Preskorn SH, Ayd FJ (eds). Principles and Practice of Psychopharmacotherapy, 2nd edn. Williams & Wilkins: Baltimore; (1997), pp 243–356.
Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA (2015). Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology 232: 3123–3133.
Jett JD, Morilak DA (2013). Too much of a good thing: blocking noradrenergic facilitation in medial prefrontal cortex prevents the detrimental effects of chronic stress on cognition. Neuropsychopharmacology 38: 585–595.
Knight ZA, Tan K, Birsoy K, Schmidt S, Garrison JL, Wysocki RW et al (2012). Molecular profiling of activated neurons by phosphorylated ribosome capture. Cell 151: 1126–1137.
Koolhaas JM, Korte SM, De Boer SF, Van der Vegt BJ, Van Reenen CG, Hopster H et al (1999). Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev 23: 925–935.
Lapiz MDS, Morilak DA (2006). Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137: 1039–1049.
Mathews A, Mackintosh B (1998). A cognitive model of selective processing in anxiety. Cognit Ther Res 22: 539–560.
McLean CP, Foa EB (2013). Dissemination and implementation of prolonged exposure therapy for posttraumatic stress disorder. J Anxiety Disord 27: 788–792.
McNally RJ (2007). Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clin Psychol Rev 27: 750–759.
Mueller D, Porter JT, Quirk GJ (2008). Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J Neurosci 28: 369–375.
Nemeroff CB, Helm CM, Thase ME, Klein DN, Rush AJ, Schatzberg AF et al (2003). Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci USA 100: 14293–14296.
Nutt DJ (2010). Rationale for, barriers to, and appropriate medication for the long-term treatment of depression. J Clin Psychiatry 71: e02.
Paxinos G, Watson C The Rat Brain in Stereotaxic Coordinates, 2nd edn. Academic Press: San Diego; (1986).
Quirk GJ, Garcia R, González-Lima F (2006). Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry 60: 337–343.
Richter JD, Klann E (2009). Making synaptic plasticity and memory last: mechanisms of translational regulation. Genes Dev 23: 1–11.
Ritchey M, Dolcos F, Eddington KM, Strauman TJ, Cabeza R (2011). Neural correlates of emotional processing in depression: changes with cognitive behavioral therapy and predictors of treatment response. J Psychiatr Res 45: 577–587.
Rogers MA, Kasai K, Koji M, Fukuda R, Iwanami A, Nakagome K et al (2004). Executive and prefrontal dysfunction in unipolar depression: a review of neuropsychological and imaging evidence. Neurosci Res 50: 1–11.
Rosenblum K, Meiri N, Dudai Y (1993). Taste memory: the role of protein synthesis in gustatory cortex. Behav Neural Biol 59: 49–56.
Roth MK, Bingham B, Shah A, Joshi A, Frazer A, Strong R et al (2012). Effects of chronic plus acute prolonged stress on measures of coping style, anxiety, and evoked HPA-axis reactivity. Neuropharmacology 63: 1118–1126.
Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ (2004). Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci 24: 5704–5710.
Shah AA, Sjuvold T, Treit D (2004). Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated plus-maze and attenuates shock-probe burying in rats. Brain Res 1028: 112–115.
Sheehan TP, Chambers RA, Russel DS (2004). Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Rev 46: 71–117.
Sheline YI (2003). Neuroimaging studies of mood disorder effects on the brain. Biol Psychiatry 54: 338–352.
Sotres-Bayon F, Bush DE, LeDoux JE (2004). Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn Mem 11: 525–535.
Straub J, Plener PL, Sproeber N, Sprenger L, Koelch MG, Groen G et al (2015). Neural correlates of successful psychotherapy of depression in adolescents. J Affect Disord 183: 239–246.
Tedesco V, Roquet RF, DeMis J, Chiamulera C, Monfils MH (2014). Extinction, applied after retrieval of auditory fear memory, selectively increases zinc-finger protein 268 and phosphorylated ribosomal protein S6 expression in prefrontal cortex and lateral amygdala. Neurobiol Learn Mem 115: 78–85.
Treit D, Pesold C, Rotzinger S (1993). Dissociating the anti-fear effects of septal and amygdaloid lesions using two pharmacologically validated models of rat anxiety. Behav Neurosci 107: 770–785.
Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J et al (2013). Cognitive behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 381: 375–384.
Yoshimura S, Okamoto Y, Onoda K, Matsunaga M, Okada G, Kunisato Y et al (2014). Cognitive behavioral therapy for depression changes medial prefrontal and ventral anterior cingulate cortex activity associated with self-referential processing. Soc Cogn Affect Neurosci 9: 487–493.
Acknowledgements
We thank Lauren Hatherall, Jeri Silva, Suphada Lertphinyowong, and Kathleen Winters for technical assistance. This work was supported by Research Grant MH072672 from the National Institute of Mental Health, T32 Training Grant NS082145 from the National Institutes of Health, a fellowship awarded through Grant TL1 TR001119 from the National Center for Advancing Translational Sciences, National Institutes of Health, and a grant from the William and Ella Owens Medical Research Foundation, none of which had any role in study design, data collection, analysis or interpretation, or in the preparation or decision to submit this paper for publication.
Author contributions
EA Fucich participated in experimental design and planning, conduct of the experiments, data analysis and interpretation, and writing and editing the manuscript. D Paredes participated in conduct of the experiments, data analysis, and editing of the manuscript. DA Morilak participated in experimental design and planning, data analysis and interpretation, writing and editing the manuscript, and provided laboratory resources and oversight for the experiments. All authors have read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fucich, E., Paredes, D. & Morilak, D. Therapeutic Effects of Extinction Learning as a Model of Exposure Therapy in Rats. Neuropsychopharmacol 41, 3092–3102 (2016). https://doi.org/10.1038/npp.2016.127
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.127
This article is cited by
-
Infralimbic BDNF signaling is necessary for the beneficial effects of extinction on set shifting in stressed rats
Neuropsychopharmacology (2022)
-
Vortioxetine reverses medial prefrontal cortex-mediated cognitive deficits in male rats induced by castration as a model of androgen deprivation therapy for prostate cancer
Psychopharmacology (2019)
-
β-Adrenoceptor Blockade in the Basolateral Amygdala, But Not the Medial Prefrontal Cortex, Rescues the Immediate Extinction Deficit
Neuropsychopharmacology (2017)