Abstract
The basal forebrain (BF) cholinergic neurons have long been thought to be involved in behavioral wakefulness and cortical activation. However, owing to the heterogeneity of BF neurons and poor selectivity of traditional methods, the precise role of BF cholinergic neurons in regulating the sleep–wake cycle remains unclear. We investigated the effects of cell-selective manipulation of BF cholinergic neurons on the sleep–wake behavior and electroencephalogram (EEG) power spectrum using the pharmacogenetic technique, the ‘designer receptors exclusively activated by designer drugs (DREADD)’ approach, and ChAT-IRES-Cre mice. Our results showed that activation of BF cholinergic neurons expressing hM3Dq receptors significantly and lastingly decreased the EEG delta power spectrum, produced low-delta non-rapid eye movement sleep, and slightly increased wakefulness in both light and dark phases, whereas inhibition of BF cholinergic neurons expressing hM4Di receptors significantly increased EEG delta power spectrum and slightly decreased wakefulness. Next, the projections of BF cholinergic neurons were traced by humanized Renilla green fluorescent protein (hrGFP). Abundant and highly dense hrGFP-positive fibers were observed in the secondary motor cortex and cingulate cortex, and sparse hrGFP-positive fibers were observed in the ventrolateral preoptic nucleus, a known sleep-related structure. Finally, we found that activation of BF cholinergic neurons significantly increased c-Fos expression in the secondary motor cortex and cingulate cortex, but decreased c-Fos expression in the ventrolateral preoptic nucleus. Taken together, these findings reveal that the primary function of BF cholinergic neurons is to inhibit EEG delta activity through the activation of cerebral cortex, rather than to induce behavioral wakefulness.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Anaclet C, Ferrari L, Arrigoni E, Bass CE, Saper CB, Lu J et al (2014). The GABAergic parafacial zone is a medullary slow wave sleep-promoting center. Nat Neurosci 17: 1217–1224.
Arrigoni E, Chamberlin NL, Saper CB, McCarley RW (2006). Adenosine inhibits basal forebrain cholinergic and noncholinergic neurons in vitro. Neuroscience 140: 403–413.
Berntson GG, Shafi R, Sarter M (2002). Specific contributions of the basal forebrain corticopetal cholinergic system to electroencephalographic activity and sleep/waking behaviour. Eur J Neurosci 16: 2453–2461.
Blanco-Centurion C, Gerashchenko D, Shiromani PJ (2007). Effects of saporin-induced lesions of three arousal populations on daily levels of sleep and wake. J Neurosci 27: 14041–14048.
Blanco-Centurion CA, Shiromani A, Winston E, Shiromani PJ (2006). Effects of hypocretin-1 in 192-IgG-saporin-lesioned rats. Eur J Neurosci 24: 2084–2088.
Cape EG, Jones BE (2000a). Effects of glutamate agonist versus procaine microinjections into the basal forebrain cholinergic cell area upon gamma and theta EEG activity and sleep-wake state. Eur J Neurosci 12: 2166–2184.
Cape EG, Manns ID, Alonso A, Beaudet A, Jones BE (2000b). Neurotensin-induced bursting of cholinergic basal forebrain neurons promotes gamma and theta cortical activity together with waking and paradoxical sleep. J Neurosci 20: 8452–8461.
Castillo-Ruiz A, Nixon JP, Smale L, Nunez AA (2010). Neural activation in arousal and reward areas of the brain in day-active and night-active grass rats. Neuroscience 165: 337–349.
Davis CJ, Clinton JM, Jewett KA, Zielinski MR, Krueger JM (2011). Delta wave power: an independent sleep phenotype or epiphenomenon? J Clin Sleep Med 7: S16–S18.
Farrell MS, Roth BL (2013). Pharmacosynthetics: reimagining the pharmacogenetic approach. Brain Res 1511: 6–20.
Freund TF, Antal M (1988). GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 336: 170–173.
Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J (2011). Reassessment of the structural basis of the ascending arousal system. J Comp Neurol 519: 933–956.
Gallopin T, Fort P, Eggermann E, Cauli B, Luppi PH, Rossier J et al (2000). Identification of sleep-promoting neurons in vitro. Nature 404: 992–995.
Gritti I, Henny P, Galloni F, Mainville L, Mariotti M, Jones BE (2006). Stereological estimates of the basal forebrain cell population in the rat, including neurons containing choline acetyltransferase, glutamic acid decarboxylase or phosphate-activated glutaminase and colocalizing vesicular glutamate transporters. Neuroscience 143: 1051–1064.
Hallanger AE, Levey AI, Lee HJ, Rye DB, Wainer BH (1987). The origins of cholinergic and other subcortical afferents to the thalamus in the rat. J Comp Neurol 262: 105–124.
Han Y, Shi YF, Xi W, Zhou R, Tan ZB, Wang H et al (2014). Selective activation of cholinergic basal forebrain neurons induces immediate sleep-wake transitions. Curr Biol 24: 693–698.
Hassani OK, Lee MG, Henny P, Jones BE (2009). Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci 29: 11828–11840.
Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB et al (2005). Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci 8: 858–859.
Irmak SO, de Lecea L (2014). Basal forebrain cholinergic modulation of sleep transitions. Sleep 37: 1941–1951.
Jackson A, Spinks RL, Freeman TC, Wolpert DM, Lemon RN (2002). Rhythm generation in monkey motor cortex explored using pyramidal tract stimulation. J Physiol 541: 685–699.
Jones BE (2004). Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog Brain Res. 145: 157–169.
Jones BE (2005). From waking to sleeping: neuronal and chemical substrates. Trends Pharmacol Sci 26: 578–586.
Kaur S, Junek A, Black MA, Semba K (2008). Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci 28: 491–504.
Lacey MG, Gooding-Williams G, Prokic EJ, Yamawaki N, Hall SD, Stanford IM et al (2014). Spike firing and IPSPs in layer V pyramidal neurons during beta oscillations in rat primary motor cortex (M1) in vitro. PLoS One 9: e85109.
Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE et al (2011). Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci 31: 10067–10075.
Losier BJ, Semba K (1993). Dual projections of single cholinergic and aminergic brainstem neurons to the thalamus and basal forebrain in the rat. Brain Res 604: 41–52.
Manfridi A, Brambilla D, Mancia M (1999). Stimulation of NMDA and AMPA receptors in the rat nucleus basalis of Meynert affects sleep. Am J Physiol 277: R1488–R1492.
Metherate R, Cox CL, Ashe JH (1992). Cellular bases of neocortical activation: modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci 12: 4701–4711.
Pace-Schott EF, Hobson JA (2002). The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci 3: 591–605.
Paxinos GT, Franklin K (2001) The Mouse Brain in Stereotaxic Coordinates, 2nd edn. Academic Press: San Diego.
Pita-Almenar JD, Yu D, Lu HC, Beierlein M (2014). Mechanisms underlying desynchronization of cholinergic-evoked thalamic network activity. J Neurosci 34: 14463–14474.
Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW (1997). Role of adenosine in behavioral state modulation: a microdialysis study in the freely moving cat. Neuroscience 79: 225–235.
Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y (2008). Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci 28: 8462–8469.
Qu WM, Xu XH, Yan MM, Wang YQ, Urade Y, Huang ZL (2010). Essential role of dopamine D2 receptor in the maintenance of wakefulness, but not in homeostatic regulation of sleep, in mice. J Neurosci 30: 4382–4389.
Qu WM, Yue XF, Sun Y, Fan K, Chen CR, Hou YP et al (2012). Honokiol promotes non-rapid eye movement sleep via the benzodiazepine site of the GABA(A) receptor in mice. Br J Pharmacol 167: 587–598.
Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE et al (2011). Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 13: 195–204.
Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T (2011). Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One 6: e20360.
Scammell TE, Estabrooke IV, McCarthy MT, Chemelli RM, Yanagisawa M, Miller MS et al (2000). Hypothalamic arousal regions are activated during modafinil-induced wakefulness. J Neurosci 20: 8620–8628.
Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA et al (2001). An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience 107: 653–663.
Semba K (2000). Multiple output pathways of the basal forebrain: organization, chemical heterogeneity, and roles in vigilance. Behav Brain Res 115: 117–141.
Steriade M, Domich L, Oakson G, Deschenes M (1987). The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol 57: 260–273.
Sun YG, Pita-Almenar JD, Wu CS, Renger JJ, Uebele VN, Lu HC et al (2013). Biphasic cholinergic synaptic transmission controls action potential activity in thalamic reticular nucleus neurons. J Neurosci 33: 2048–2059.
Szymusiak R, Alam N, Steininger TL, McGinty D (1998). Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res 803: 178–188.
Takahashi K, Lin JS, Sakai K (2009). Characterization and mapping of sleep-waking specific neurons in the basal forebrain and preoptic hypothalamus in mice. Neuroscience 161: 269–292.
von Krosigk M, Bal T, McCormick DA (1993). Cellular mechanisms of a synchronized oscillation in the thalamus. Science 261: 361–364.
Yang C, McKenna JT, Zant JC, Winston S, Basheer R, Brown RE (2014). Cholinergic neurons excite cortically projecting basal forebrain GABAergic neurons. J Neurosci 34: 2832–2844.
Yoder RM, Pang KC (2005). Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus 15: 381–392.
Zaborszky L, Duque A (2000). Local synaptic connections of basal forebrain neurons. Behav Brain Res 115: 143–158.
Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B et al (2011). Cell type-specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods 8: 745–752.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, L., Yin, D., Wang, TX. et al. Basal Forebrain Cholinergic Neurons Primarily Contribute to Inhibition of Electroencephalogram Delta Activity, Rather Than Inducing Behavioral Wakefulness in Mice. Neuropsychopharmacol 41, 2133–2146 (2016). https://doi.org/10.1038/npp.2016.13
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/npp.2016.13
This article is cited by
-
REM sleep is associated with the volume of the cholinergic basal forebrain in aMCI individuals
Alzheimer's Research & Therapy (2023)
-
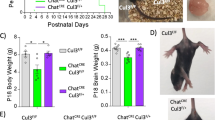
Cholinergic neurons in the basal forebrain are involved in behavioral abnormalities associated with Cul3 deficiency: Role of prefrontal cortex projections in cognitive deficits
Translational Psychiatry (2023)
-
Neuro-orchestration of sleep and wakefulness
Nature Neuroscience (2023)
-
A cluster of mesopontine GABAergic neurons suppresses REM sleep and curbs cataplexy
Cell Discovery (2022)
-
Ventral pallidal GABAergic neurons control wakefulness associated with motivation through the ventral tegmental pathway
Molecular Psychiatry (2021)