Abstract
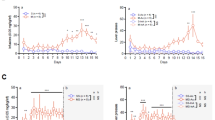
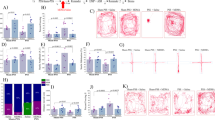
The persistent use of psychostimulant drugs, despite the detrimental outcomes associated with continued drug use, may be because of disruptions in reinforcement-learning processes that enable behavior to remain flexible and goal directed in dynamic environments. To identify the reinforcement-learning processes that are affected by chronic exposure to the psychostimulant methamphetamine (MA), the current study sought to use computational and biochemical analyses to characterize decision-making processes, assessed by probabilistic reversal learning, in rats before and after they were exposed to an escalating dose regimen of MA (or saline control). The ability of rats to use flexible and adaptive decision-making strategies following changes in stimulus–reward contingencies was significantly impaired following exposure to MA. Computational analyses of parameters that track choice and outcome behavior indicated that exposure to MA significantly impaired the ability of rats to use negative outcomes effectively. These MA-induced changes in decision making were similar to those observed in rats following administration of a dopamine D2/3 receptor antagonist. These data use computational models to provide insight into drug-induced maladaptive decision making that may ultimately identify novel targets for the treatment of psychostimulant addiction. We suggest that the disruption in utilization of negative outcomes to adaptively guide dynamic decision making is a new behavioral mechanism by which MA rigidly biases choice behavior.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Albers DS, Sonsalla PK (1995). Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther 275: 111.
Ali SF, Newport GD, Holson RR, Slikker W, Bowyer JF (1994). Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res 658: 33–38.
Barraclough DJ, Conroy ML, Lee D (2004). Prefrontal cortex and decision making in a mixed-strategy game. Nat Neurosci 7: 404–410.
Bechara A (2005). Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci 8: 1458–1463.
Bourne JA (2001). SCH 23390: the first selective dopamine D1-like receptor antagonist. CNS Drug Rev 7: 399–414..
Chiu PH, Lohrenz TM, Montague PR (2008). Smokers’ brains compute, but ignore, a fictive error signal in a sequential investment task. Nat Neurosci 11: 514–520.
Cho AK, Melega WP, Kuczenski R, Segal DS (2001). Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse 39: 161–166.
Collins P, Broekkamp CL, Jenner P, Marsden CD (1991). Drugs acting at D-1 and D-2 dopamine receptors induce identical purposeless chewing in rats which can be differentiated by cholinergic manipulation. Psychopharmacology (Berl) 103: 503–512.
Costa VD, Tran VL, Turchi J, Averbeck BB (2015). Reversal learning and dopamine: a Bayesian perspective. J Neurosci 35: 2407–2416.
Cox BM, Cope ZA, Parsegian A, Floresco SB, Aston-Jones G, See RE (2016). Chronic methamphetamine self-administration alters cognitive flexibility in male rats. Psychopharmacology (Berl) 233: 2319–2327.
Dalton GL, Wang NY, Phillips AG, Floresco SB (2016). Multifaceted contributions by different regions of the orbitofrontal and medial prefrontal cortex to probabilistic reversal learning. J Neurosci 36: 1996–2006.
Dayan P (2009). Dopamine, reinforcement learning, and addiction. Pharmacopsychiatry 42: S56–S65.
Ersche KD, Gillan CM, Jones PS, Williams GB, Ward LH, Luijten M et al (2016). Carrots and sticks fail to change behavior in cocaine addiction. Science 352: 1468–1471.
Ersche KD, Roiser JP, Robbins TW, Sahakian BJ (2008). Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacol 197: 421–431.
Everitt BJ, Robbins TW (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489.
Fillmore MT, Rush CR (2006). Polydrug abusers display impaired discrimination-reversal learning in a model of behavioural control. J Psychopharmacol 20: 24–32.
Frank MJ (2004). By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science (80-) 306: 1940–1943.
Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, London ED (2011). Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology 36: 950–959.
Goldstein RZ, Volkow ND (2002). Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159: 1642–1652.
Groman SM, Jentsch JD (2012). Cognitive control and the dopamine D(2)-like receptor: a dimensional understanding of addiction. Depress Anxiety 29: 295–306.
Groman SM, Lee B, Seu E, James AS, Feiler K, Mandelkern MA et al (2012). Dysregulation of D2-mediated dopamine transmission in monkeys after chronic escalating methamphetamine exposure. J Neurosci 32: 5843–5852.
Groman SM, Smith NJ, Petrullli JR, Massi B, Chen L, Ropchan J et al (2016). Dopamine D3 receptor availability is associated with inflexible decision making. J Neurosci 36: 6732–6741.
Han E, Paulus MP, Wittmann M, Chung H, Song JM (2011). Hair analysis and self-report of methamphetamine use by methamphetamine dependent individuals. J Chromatogr B Anal Technol Biomed Life Sci 879: 541–547.
Harlé KM, Zhang S, Schiff M, Mackey S, Paulus MP, Yu AJ (2015). Altered statistical learning and decision-making in methamphetamine dependence: evidence from a two-armed bandit task. Front Psychol 6: 1910.
Higa KK, Young JW, Ji B, Nichols DE, Geyer MA, Zhou X (2017). Striatal dopamine D1 receptor suppression impairs reward-associative learning. Behav Brain Res 323: 100–110.
Huys QJM, Lally N, Faulkner P, Eshel N, Seifritz E, Gershman SJ et al (2015). Interplay of approximate planning strategies. Proc Natl Acad Sci USA 112: 3098–3103.
Ito M, Doya K (2009). Validation of decision-making models and analysis of decision variables in the rat basal ganglia. J Neurosci 29: 9861–9874.
Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ et al (2010). Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology 35: 505–514.
Jenni NL, Larkin JD, Floresco SB (2017). Prefrontal dopamine D1 and D2 receptors regulate dissociable aspects of decision making via distinct ventral striatal and amygdalar circuits. J Neurosci 37: 6200–6213.
Jentsch JD, Olausson P, De La Garza R 2nd, Taylor JR (2002). Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology 26: 183–190.
Jentsch JD, Taylor JR (1999). Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146: 373–390.
Keeler JF, Pretsell DO, Robbins TW (2014). Functional implications of dopamine D1 vs. D2 receptors: A “prepare and select” model of the striatal direct vs. indirect pathways. Neuroscience 282: 156–175.
Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED (2014). Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry 71: 812–820.
Kubler A, Murphy K, Garavan H (2005). Cocaine dependence and attention switching within and between verbal and visuospatial working memory. Eur J Neurosci 21: 1984–1992.
Lau B, Glimcher PW (2005). Dynamic response-by-response models of matching behavior in rhesus monkeys. J Exp Anal Behav 84: 555–579.
Lee B, Groman S, London ED, Jentsch JD (2007). Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology 32: 2125–2134.
Ljungberg T, Enquist M (1990). Effects of dopamine D-1 and D-2 antagonists on decision making by rats: no reversal of neuroleptic-induced attenuation by scopolamine. J Neural Transm 82: 167–179.
Lucantonio F, Stalnaker TA, Shaham Y, Niv Y, Schoenbaum G (2012). The impact of orbitofrontal dysfunction on cocaine addiction. Nat Neurosci 15: 358–366.
Mattingly BA, Rowlett JK, Lovell G (1993). Effects of daily SKF 38393, quinpirole, and SCH 23390 treatments on locomotor activity and subsequent sensitivity to apomorphine. Psychopharmacology 110: 320–326.
Montague PR, Dolan RJ, Friston KJ, Dayan P (2012). Computational psychiatry. Trends Cogn Sci 16: 72–80.
O’Neil ML, Kuczenski R, Segal DS, Cho AK, Lacan G, Melega WP (2006). Escalating dose pretreatment induces pharmacodynamic and not pharmacokinetic tolerance to a subsequent high-dose methamphetamine binge. Synapse 60: 465–473.
Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J et al (2010). Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci 30: 7749–7753.
Parker NF, Cameron CM, Taliaferro JP, Lee J, Choi JY, Davidson TJ et al (2016). Reward and choice encoding in terminals of midbrain dopamine neurons depends on striatal target. Nat Neurosci 19: 845–854.
Parvaz MA, Konova AB, Proudfit GH, Dunning JP, Malaker P, Moeller SJ et al (2015). Impaired neural response to negative prediction errors in cocaine addiction. J Neurosci 35: 1872–1879.
Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA (2003). Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol Psychiatry 53: 65–74.
Redish AD (2004). Addiction as a computational process gone awry. Science 306: 1944–1947.
Redish AD, Jensen S, Johnson A (2008). A unified framework for addiction: vulnerabilities in the decision process. Behav Brain Sci 31: 415–487.
Rominger A, Cumming P, Xiong G, Koller G, Böning G, Wulff M et al (2012). [18F]fallypride PET measurement of striatal and extrastriatal dopamine D2/3 receptor availability in recently abstinent alcoholics. Addict Biol 17: 490–503.
Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B (2004). Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci 19: 1997–2002.
Sebold M, Deserno L, Nebe S, Schad DJ, Garbusow M, Hägele C et al (2014). Model-based and model-free decisions in alcohol dependence. Neuropsychobiology 70: 122–131.
Segal DS, Kuczenski R, O’Neil ML, Melega WP, Cho AK (2003). Escalating dose methamphetamine pretreatment alters the behavioral and neurochemical profiles associated with exposure to a high-dose methamphetamine binge. Neuropsychopharmacology 28: 1730–1740.
Sjoerds Z, de Wit S, van den Brink W, Robbins TW, Beekman ATF, Penninx BWJH et al (2013). Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl Psychiatry 3: e337.
Stalnaker TA, Takahashi Y, Roesch MR, Schoenbaum G (2009). Neural substrates of cognitive inflexibility after chronic cocaine exposure. Neuropharmacology 56: 63–72.
Stolyarova A, O’Dell SJ, Marshall JF, Izquierdo A (2014). Positive and negative feedback learning and associated dopamine and serotonin transporter binding after methamphetamine. Behav Brain Res 271: 195–202.
Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F et al (2006). High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry 63: 999–1008.
Acknowledgements
This study was supported by public health service grants DA041480 and DA043443, NARSAD, the Charles B.G. Murphy Fund, and the State of CT Department of Mental Health Services.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Groman, S., Rich, K., Smith, N. et al. Chronic Exposure to Methamphetamine Disrupts Reinforcement-Based Decision Making in Rats. Neuropsychopharmacol. 43, 770–780 (2018). https://doi.org/10.1038/npp.2017.159
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/npp.2017.159
This article is cited by
-
The mesocorticolimbic system in stimulant use disorder
Molecular Psychiatry (2025)
-
Exercise-mediated modulation of hippocampal apoptotic gene expression and behavioral outcomes in methamphetamine-dependent rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2025)
-
Expertise increases planning depth in human gameplay
Nature (2023)
-
Exploring new histone deacetylase 6 inhibitors and their effects on reversing the α-tubulin deacetylation and cell morphology changes caused by methamphetamine
Archives of Pharmacal Research (2023)
-
Adolescent reinforcement-learning trajectories predict cocaine-taking behaviors in adult male and female rats
Psychopharmacology (2022)