Abstract
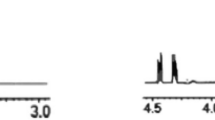
Substrate activity screening (SAS) is a fragment-based method for the rapid development of novel substrates and their conversion into non-peptidic inhibitors of Cys and Ser proteases. The method consists of three steps: (i) a library of N-acyl aminocoumarins with diverse, low-molecular-weight N-acyl groups is screened to identify protease substrates using a simple fluorescence-based assay; (ii) the identified N-acyl aminocoumarin substrates are optimized by rapid analog synthesis and evaluation; and (iii) the optimized substrates are converted into inhibitors by direct replacement of the aminocoumarin with known mechanism–based pharmacophores. This protocol describes a general procedure for the solid-phase synthesis of a library of N-acyl aminocoumarin substrates and the screening procedure to identify weak binding substrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wood, W.J.L., Patterson, A.W., Tsuruoka, H., Jain, R.K. & Ellman, J.A. Substrate activity screening: a fragment-based method for the rapid identification of nonpeptidic protease inhibitors. J. Am. Chem. Soc. 127, 15521–15527 (2005).
Patterson, A.W. et al. Identification of selective, nonpeptidic nitrile inhibitors of cathepsin S using the substrate activity screening method. J. Med. Chem. 49, 6298–6307 (2006).
Salisbury, C.M. & Ellman, J.A. Rapid identification of potent nonpeptidic serine protease inhibitors. Chembiochem. 7, 1034–1037 (2006).
Erlanson, D.A., McDowell, R.S. & O'Brien, T. Fragment-based drug discovery. J. Med. Chem. 47, 3463–3482 (2004).
Keseru, G.M. & Makara, G.M. Hit discovery and hit-to-lead approaches. Drug Discov. Today 11, 741–748 (2006).
Link, J.O. & Zipfel, S. Advances in cathepsin S inhibitor design. Curr. Opin. Drug Discov. Devel. 9, 471–482 (2006).
Leung-Toung, R. et al. Thiol proteases: inhibitors and potential therapeutic targets. Curr. Med. Chem. 13, 547–581 (2006).
Villhauer, E.B. et al. 1-[[(3-hydroxy-1-adamantyl)amino]acetyl]-2-cyano-(S)-pyrrolidine: a potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 46, 2774–2789 (2003).
Choe, Y. et al. Substrate profiling of cysteine proteases using a combinatorial peptide library identifies functionally unique specificities. J. Biol. Chem. 281, 12824–12832 (2006).
Mader, M.M. & Bartlett, P.A. Binding energy and catalysis: the implications for transition-state analogs and catalytic antibodies. Chem. Rev. 97, 1281–1302 (1997).
Thornberry, N.A. et al. A combinatorial approach for determining protease specificities: application to interleukin-1â converting enzyme (ICE). Chem. Biol. 4, 149–155 (1997).
Lesner, A., Kupryszewski, G. & Rolka, K. Chromogenic substrates of bovine β-trypsin: the influence of an amino acid residue in P1 position on their interaction with the enzyme. Biochem. Biophys. Res. Commun. 285, 1350–1353 (2001).
Leung, D., Abbenante, G. & Fairlie, D.P. Protease inhibitors: current status and future prospects. J. Med. Chem. 43, 305–341 (2000).
Loser, R., Schilling, K., Dimmig, E. & Gutschow, M. Interaction of papain-like cysteine proteases with dipeptide-derived nitriles. J. Med. Chem. 48, 7688–7707 (2005).
Liu, H. et al. Design and synthesis of arylaminoethyl amides as noncovalent inhibitors of cathepsin S. Part 1. Bioorg. Med. Chem. Lett. 15, 4979–4984 (2005).
Powers, J.C., Asgian, J.L., Ekici, O.D. & James, K.E. Irreversible inhibitors of serine, cysteine, and threonine proteases. Chem. Rev. 102, 4639–4750 (2002).
Maly, D.J. et al. Expedient solid-phase synthesis of fluorogenic protease substrates using the 7-amino-4-carbamoylmethylcoumarin (ACC) fluorophore. J. Org. Chem. 67, 910–915 (2002).
Bunin, B.A. Fmoc quantitation. In The Combinatorial Index 5.2.2 219–220 (Academic Press, San Diego, USA, 1998).
Peptide synthesis protocols. In Novabiochem Catalog 3.1–3.10 (EMD Biosciences, San Diego, USA, 2004/2005).
Acknowledgements
The authors would like to thank the National Institutes of Health (GM54051) for support of this work. A.W.P. greatly appreciates an American Chemical Society Medicinal Chemistry Graduate Student Fellowship sponsored by Bristol-Myers Squibb.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Patterson, A., Wood, W. & Ellman, J. Substrate activity screening (SAS): a general procedure for the preparation and screening of a fragment-based non-peptidic protease substrate library for inhibitor discovery. Nat Protoc 2, 424–433 (2007). https://doi.org/10.1038/nprot.2007.28
Published:
Issue date:
DOI: https://doi.org/10.1038/nprot.2007.28
This article is cited by
-
Methylated-antibody affinity purification to improve proteomic identification of plant RNA polymerase Pol V complex and the interacting proteins
Scientific Reports (2017)
-
Synthesis of a HyCoSuL peptide substrate library to dissect protease substrate specificity
Nature Protocols (2017)
-
Catalytic activation of pre-substrates via dynamic fragment assembly on protein templates
Nature Communications (2014)
-
New approaches for dissecting protease functions to improve probe development and drug discovery
Nature Structural & Molecular Biology (2012)