Abstract
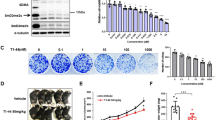
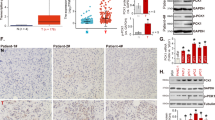
The mRNA cap-binding protein eIF4E (eukaryotic translation initiation factor 4E) permits ribosome recruitment to capped mRNAs, and its phosphorylated form has an important role in cell transformation. The oncogenic function of eIF4E is, however, antagonised by the hypophosphorylated forms of the inhibitory eIF4E-binding proteins 1 and 2. eIF4E-binding protein 1 and 2 (4E-BP1 and 2) are two major targets of the protein kinase mTOR, and are essential for the antiproliferative effects of mTOR inhibitors. Herein, we report that pancreas expresses specifically and massively 4E-BP1 (4E-BP2 is nearly undetectable). However, 4E-BP1 expression is extinguished in more than half of the human pancreatic ductal adenocarcinomas (PDAC). 4E-BP1 shutoff is recapitulated in a mouse genetic model of PDAC, which is based on a pancreas-specific mutation of Kras, the more frequently mutated oncogene in human pancreatic tumours. 4E-BP1 downregulation enhances eIF4E phosphorylation and facilitates pancreatic cancer cell proliferation in vitro and tumour development in vivo. Furthermore, 4E-BP1 loss combined with the absence of 4E-BP2 renders eIF4E phosphorylation, protein synthesis and cell proliferation resistant to mTOR inhibition. However, proliferation can be better limited by a recently developed compound that mimics the function of 4E-BP1 and 2 independently of mTOR inhibition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sonenberg N, Hinnebusch AG . Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 2009; 136: 731–745.
Blagden SP, Willis AE . The biological and therapeutic relevance of mRNA translation in cancer. Nat Rev Clin Oncol 2011; 8: 280–291.
Waskiewicz AJ, Johnson JC, Penn B, Mahalingam M, Kimball SR, Cooper JA . Phosphorylation of the cap-binding protein eukaryotic translation initiation factor 4E by protein kinase Mnk1 in vivo. Mol Cell Biol 1999; 19: 1871–1880.
Pyronnet S, Imataka H, Gingras AC, Fukunaga R, Hunter T, Sonenberg N . Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J 1999; 18: 270–279.
Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci USA 2010; 107: 14134–14139.
Ueda T, Sasaki M, Elia AJ, Chio II, Hamada K, Fukunaga R et al. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc Natl Acad Sci USA 2010; 107: 13984–13990.
Martineau Y, Azar R, Bousquet C, Pyronnet S . Anti-oncogenic potential of the eIF4E-binding proteins. Oncogene 2013; 32: 671–677.
Wander SA, Hennessy BT, Slingerland JM . Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J Clin Invest 2011; 121: 1231–1241.
Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol 2009; 27: 193–198.
Hidalgo M . Pancreatic cancer. N Engl J Med 2010; 362: 1605–1617.
Bragado MJ, Tashiro M, Williams JA . Regulation of the initiation of pancreatic digestive enzyme protein synthesis by cholecystokinin in rat pancreas in vivo. Gastroenterology 2000; 119: 1731–1739.
Sans MD, Tashiro M, Vogel NL, Kimball SR, D'Alecy LG, Williams JA . Leucine activates pancreatic translational machinery in rats and mice through mTOR independently of CCK and insulin. J Nutr 2006; 136: 1792–1799.
Tsukiyama-Kohara K, Poulin F, Kohara M, DeMaria CT, Cheng A, Wu Z et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat Med 2001; 7: 1128–1132.
Tsukiyama-Kohara K, Vidal SM, Gingras AC, Glover TW, Hanash SM, Heng H et al. Tissue distribution, genomic structure, and chromosome mapping of mouse and human eukaryotic initiation factor 4E-binding proteins 1 and 2. Genomics 1996; 38: 353–363.
Morris JPt, Wang SC, Hebrok M . KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer 2010; 10: 683–695.
Pin CL, Rukstalis JM, Johnson C, Konieczny SF . The bHLH transcription factor Mist1 is required to maintain exocrine pancreas cell organization and acinar cell identity. J Cell Biol 2001; 155: 519–530.
Bidinosti M, Ran I, Sanchez-Carbente MR, Martineau Y, Gingras AC, Gkogkas C et al. Postnatal deamidation of 4E-BP2 in brain enhances its association with raptor and alters kinetics of excitatory synaptic transmission. Mol cell 2010; 37: 797–808.
Adesso L, Calabretta S, Barbagallo F, Capurso G, Pilozzi E, Geremia R et al. Gemcitabine triggers a pro-survival response in pancreatic cancer cells through activation of the MNK2/eIF4E pathway. Oncogene 2013; 32: 2848–2857.
Azar R, Alard A, Susini C, Bousquet C, Pyronnet S . 4E-BP1 is a target of Smad4 essential for TGFbeta-mediated inhibition of cell proliferation. EMBO J 2009; 28: 3514–3522.
Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 2009; 7: e38.
Schmidt EK, Clavarino G, Ceppi M, Pierre P . SUnSET, a nonradioactive method to monitor protein synthesis. Nat Methods 2009; 6: 275–277.
Averous J, Fonseca BD, Proud CG . Regulation of cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene 2008; 27: 1106–1113.
Topisirovic I, Ruiz-Gutierrez M, Borden KL . Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res 2004; 64: 8639–8642.
Cencic R, Desforges M, Hall DR, Kozakov D, Du Y, Min J et al. Blocking eIF4E-eIF4G Interaction as a Strategy To Impair Coronavirus Replication. J Virol 2011; 85: 6381–6389.
Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012; 491: 399–405.
Armengol G, Rojo F, Castellvi J, Iglesias C, Cuatrecasas M, Pons B et al. 4E-binding protein 1: a key molecular ‘funnel factor’ in human cancer with clinical implications. Cancer Res 2007; 67: 7551–7555.
O'Reilly KE, Warycha M, Davies MA, Rodrik V, Zhou XK, Yee H et al. Phosphorylated 4E-BP1 is associated with poor survival in melanoma. Clin Cancer Res 2009; 15: 2872–2878.
Rojo F, Najera L, Lirola J, Jimenez J, Guzman M, Sabadell MD et al. 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin Cancer Res 2007; 13: 81–89.
Alain T, Morita M, Fonseca BD, Yanagiya A, Siddiqui N, Bhat M et al. eIF4E/4E-BP ratio predicts the efficacy of mTOR targeted therapies. Cancer Res 2012; 72: 6468–6476.
Cencic R, Hall DR, Robert F, Du Y, Min J, Li L et al. Reversing chemoresistance by small molecule inhibition of the translation initiation complex eIF4F. Proc Natl Acad Sci USA 2011; 108: 1046–1051.
Dowling RJ, Topisirovic I, Alain T, Bidinosti M, Fonseca BD, Petroulakis E et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science (New York, NY) 2010; 328: 1172–1176.
Mamane Y, Petroulakis E, Martineau Y, Sato TA, Larsson O, Rajasekhar VK et al. Epigenetic activation of a subset of mRNAs by eIF4E explains its effects on cell proliferation. PLoS ONE 2007; 2: e242.
Acknowledgements
This work was supported by grants from INSERM-Université Paul Sabatier and from La LIGUE (‘Comités de Hautes-Pyrénées et de Lot-et-Garonne’ and ‘Equipes Labellisées’ programs) to SP. Yvan Martineau was a recipient of Fondation de France and FRM post-doctoral fellowships. Rania Azar, David Müller and Charline Lasfargues were recipients of doctoral fellowships from ARC, La Ligue Nationale Contre le Cancer and CFP, respectively. Rodica Anesia was supported by the RITC foundation. We thank Dr Marlène Dufresne, Dr Véronique Gigoux and Mr Pascal Clerc for their help in characterising Pdx1Cre-KrasG12D mice. We thank Professor Nahum Sonenberg for anti-eIF4GI antibody and Dr Philippe Pierre for the anti-puromycin antibody.
Author contributions:YM and RA designed, performed and analysed the experiments, and wrote the report. DM, CL, SK and RAn performed experiments and analysed the data. JP provided reagents. CB and SP designed and analysed experiments, and wrote the report.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Martineau, Y., Azar, R., Müller, D. et al. Pancreatic tumours escape from translational control through 4E-BP1 loss. Oncogene 33, 1367–1374 (2014). https://doi.org/10.1038/onc.2013.100
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2013.100
Keywords
This article is cited by
-
Targeting eIF4F translation initiation complex with SBI-756 sensitises B lymphoma cells to venetoclax
British Journal of Cancer (2021)
-
Monitoring flux in signalling pathways through measurements of 4EBP1-mediated eIF4F complex assembly
BMC Biology (2019)
-
Epigenetic downregulation of STAT6 increases HIF-1α expression via mTOR/S6K/S6, leading to enhanced hypoxic viability of glioma cells
Acta Neuropathologica Communications (2019)
-
Implication of 4E-BP1 protein dephosphorylation and accumulation in pancreatic cancer cell death induced by combined gemcitabine and TRAIL
Cell Death & Disease (2017)
-
Survival of pancreatic cancer cells lacking KRAS function
Nature Communications (2017)