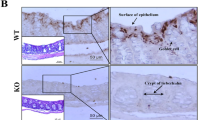
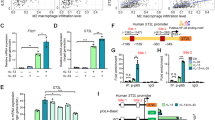
Abstract
In a model of peritoneal metastasis in immune-competent mice, we show that nuclear factor (NF)-κB inhibition in CT26 colon cancer cells prevents metastasis. NF-κB inhibition, by stable overexpression of IκB-α super-repressor, induced differential polarization of co-cultured macrophages to an M1-like anti-tumour phenotype in vitro. NF-κB-deficient cancer cell-conditioned media (CT26/IκB-α SR) induced interleukin (IL)-12 and nitric oxide (NO) synthase (inducible NO synthase (iNOS)) expression in macrophages. Control cell (CT26/EV) conditioned media induced high levels of IL-10 and arginase in macrophages. In vivo, this effect translated to reduction in metastasis in mice injected with CT26/ IκB-α SR cells and was positively associated with increased CD8+CD44+CD62L− and CD4+CD44+CD62L− effector T cells. Furthermore, inhibition of NF-κB activity induced high levels of NO in infiltrating immune cells and decreases in matrix metalloproteinase-9 expression, simultaneous with increases in tissue inhibitor of metalloproteinases 1 and 2 within tumours. CT26/IκB-α SR tumours displayed increased pro-inflammatory gene expression, low levels of angiogenesis and extensive intratumoral apoptosis, consistent with the presence of an anti-tumour macrophage phenotype. Macrophage depletion reduced tumour size in CT26/EV-injected animals and increased tumour size in CT26/IκB-α SR cells compared with untreated tumours. Our data demonstrate, for the first time, that an important implication of targeting tumour cell NF-κB is skewing of macrophage polarization to an anti-tumour phenotype. This knowledge offers novel therapeutic opportunities for anticancer treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Kianmanesh R, Scaringi S, Sabate JM, Castel B, Pons-Kerjean N, Coffin B et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg 2007; 245: 597–603.
Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol 2004; 11: 178–186.
Cintron JR, Pearl RK . Colorectal cancer and peritoneal carcinomatosis. Semin Surg Oncol 1996; 12: 267–278.
Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP . Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg 2006; 243: 212–222.
Pollard JW . Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004; 4: 71–78.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Mantovani A, Allavena P, Sica A, Balkwill F . Cancer-related inflammation. Nature 2008; 454: 436–444.
Hallam S, Escorcio-Correia M, Soper R, Schultheiss A, Hagemann T . Activated macrophages in the tumour microenvironment-dancing to the tune of TLR and NF-kappaB. J Pathol 2009; 219: 143–152.
Condeelis J, Pollard JW . Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 2006; 124: 263–266.
Balkwill F, Charles KA, Mantovani A . Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005; 7: 211–217.
Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell 2011; 19: 31–44.
Mancino A, Lawrence T . Nuclear factor-kappaB and tumor-associated macrophages. Clin Cancer Res 16: 784–789.
Bingle L, Brown NJ, Lewis CE . The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002; 196: 254–265.
De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell 2008; 14: 299–311.
Li Q, Verma IM . NF-kappaB regulation in the immune system. Nat Rev Immunol 2002; 2: 725–734.
Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004; 431: 461–466.
Lin Y, Bai L, Chen W, Xu S . The NF-kappaB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets 14: 45–55.
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004; 118: 285–296.
Toruner M, Fernandez-Zapico M, Sha JJ, Pham L, Urrutia R, Egan LJ . Antianoikis effect of nuclear factor-kappaB through up-regulated expression of osteoprotegerin, BCL-2, and IAP-1. J Biol Chem 2006; 281: 8686–8696.
Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG et al. "Re-educating" tumor-associated macrophages by targeting NF-kappaB. J Exp Med 2008; 205: 1261–1268.
Kojima M, Morisaki T, Sasaki N, Nakano K, Mibu R, Tanaka M et al. Increased nuclear factor-kB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res 2004; 24: 675–681.
Karin M, Greten FR . NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005; 5: 749–759.
Martinez FO, Sica A, Mantovani A, Locati M . Macrophage activation and polarization. Front Biosci 2008; 13: 453–461.
Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY et al. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res 2010; 70: 4840–4849.
Koppelman B, Neefjes JJ, de Vries JE, de Waal Malefyt R . Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 1997; 7: 861–871.
DeNardo DG, Andreu P, Coussens LM . Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev 29: 309–316.
Soudja SM, Ruiz AL, Marie JC, Lauvau G . Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 2012; 37: 549–562.
Camus M, Tosolini M, Mlecnik B, Pages F, Kirilovsky A, Berger A et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res 2009; 69: 2685–2693.
Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. New Engl J Med 2005; 353: 2654–2666.
Matsuzaki S, Shinozaki K, Kobayashi N, Agematsu K . Polarization of Th1/Th2 in human CD4 T cells separated by CD62L: analysis by transcription factors. Allergy 2005; 60: 780–787.
Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M et al. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med 2009; 206: 1089–1102.
Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res 2006; 66: 11238–11246.
Karin M, Cao Y, Greten FR, Li ZW . NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2002; 2: 301–310.
Mueller MM, Fusenig NE . Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004; 4: 839–849.
Zins K, Abraham D, Sioud M, Aharinejad S . Colon cancer cell-derived tumor necrosis factor-alpha mediates the tumor growth-promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer Res 2007; 67: 1038–1045.
Flemming A . Cancer: re-educating tumour-associated macrophages. Nat Rev Drug Discov 2011; 10: 177.
Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N et al. Low-dose irradiation programs macrophage differentiation to an iNOS(+)/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013; 24: 589–602.
Dunn GP, Old LJ, Schreiber RD . The immunobiology of cancer immunosurveillance and immunoediting. Immunity 2004; 21: 137–148.
Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71: 1263–1271.
Xie QW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 1992; 256: 225–228.
Ridnour LA, Windhausen AN, Isenberg JS, Yeung N, Thomas DD, Vitek MP et al. Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc Natl Acad Sci USA 2007; 104: 16898–16903.
Kopp E, Ghosh S . Inhibition of NF-kappa B by sodium salicylate and aspirin. Science 1994; 265: 956–959.
Egan LJ, Mays DC, Huntoon CJ, Bell MP, Pike MG, Sandborn WJ et al. Inhibition of interleukin-1-stimulated NF-kappaB RelA/p65 phosphorylation by mesalamine is accompanied by decreased transcriptional activity. J Biol Chem 1999; 274: 26448–26453.
Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005; 294: 47–55.
Eaden J . Review article: the data supporting a role for aminosalicylates in the chemoprevention of colorectal cancer in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2003; 18: 15–21.
Luo JL, Maeda S, Hsu LC, Yagita H, Karin M . Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell 2004; 6: 297–305.
Foran E, Garrity-Park MM, Mureau C, Newell J, Smyrk TC, Limburg PJ et al. Upregulation of DNA methyltransferase-mediated gene silencing, anchorage-independent growth, and migration of colon cancer cells by interleukin-6. Mol Cancer Res 8: 471–481.
Ryan AE, Lohan P, O'Flynn L, Treacy O, Chen X, Coleman C et al. Chondrogenic differentiation increases anti-donor immune response to allogeneic mesenchymal stem cell (MSC) transplantation. Mol Ther 2013; 22: 655–667.
O'Gorman A, Colleran A, Ryan A, Mann J, Egan LJ . Regulation of NF-kappaB responses by epigenetic suppression of IkappaBalpha expression in HCT116 intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2010; 299: G96–G105.
Acknowledgements
This work was supported by funding from the Irish Cancer Society to AER (CRF12RYA) and from the Science Foundation Ireland (04/IN3/B748) and the Irish Cancer Society to LJE.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ryan, A., Colleran, A., O'Gorman, A. et al. Targeting colon cancer cell NF-κB promotes an anti-tumour M1-like macrophage phenotype and inhibits peritoneal metastasis. Oncogene 34, 1563–1574 (2015). https://doi.org/10.1038/onc.2014.86
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2014.86
This article is cited by
-
A novel NF-κB regulator encoded by circPLCE1 inhibits colorectal carcinoma progression by promoting RPS3 ubiquitin-dependent degradation
Molecular Cancer (2021)
-
Cyclodextrin-based host-guest complexes loaded with regorafenib for colorectal cancer treatment
Nature Communications (2021)
-
Tasquinimod triggers an early change in the polarization of tumor associated macrophages in the tumor microenvironment
Journal for ImmunoTherapy of Cancer (2015)