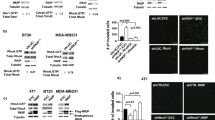
Abstract
Non-small-cell lung cancer (NSCLC) is the leading cause of cancer-related death worldwide, which is mainly due to its high risk of metastatic dissemination. One critical point of this process is the ability of cancer cells to detach from the primary tumor and migrate through the extracellular matrix; however, the underlying molecular mechanisms are not yet fully understood. In the present study, we identified the small GTPase RhoB as a key regulator of bronchial cell morphology in a three-dimensional (3D) matrix. RhoB loss, which is frequently observed during lung cancer progression, induced an epithelial–mesenchymal transition (EMT) characterized by an increased proportion of invasive elongated cells in 3D. The process was mediated by Slug induction and E-cadherin repression. In addition, downregulation of RhoB induced Akt1 activation, which in turn activated Rac1 through the guanine-exchange factor Trio to control cell shape rearrangement. Further, we provide evidence that RhoB interacted with and positively regulates phosphatase PP2A through the recruitment of its regulatory subunit B55, which was found to be crucial for Akt dephosphorylation. B55 inhibition completely suppressed RhoB-mediated PP2A regulation. Finally, we show that PP2A inactivation, by targeting either its catalytic or its regulatory B55 subunit, completely reversed RhoB-dependent morphological changes and also fully prevented the ability of RhoB to decrease the invasiveness of bronchial cells. Altogether, these results highlight a novel signaling axis and describe new molecular mechanisms that could explain the tumor suppressor role of RhoB in lung cancer. Therefore, we propose that RhoB could be responsible for early metastatic prevention by inhibiting the EMT-derived invasiveness of lung cells through the control of PP2A activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Thiery JP, Sleeman JP . Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7: 131–142.
Puisieux A, Brabletz T, Caramel J . Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 2014; 16: 488–494.
Friedl P, Alexander S . Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 2011; 147: 992–1009.
Ridley AJ . Rho GTPases and cell migration. J Cell Sci 2001; 114: 2713–2722.
Karlsson R, Pedersen ED, Wang Z, Brakebusch C . Rho GTPase function in tumorigenesis. Biochim Biophys Acta 2009; 1796: 91–98.
Vega FM, Ridley AJ . Rho GTPases in cancer cell biology. FEBS Lett 2008; 582: 2093–2101.
Grise F, Sena S, Bidaud-Meynard A, Baud J, Hiriart JB, Makki K et al. Rnd3/RhoE Is down-regulated in hepatocellular carcinoma and controls cellular invasion. Hepatology 2012; 55: 1766–1775.
Bustelo XR . Intratumoral stages of metastatic cells: a synthesis of ontogeny, Rho/Rac GTPases, epithelial-mesenchymal transitions, and more. Bioessays 2012; 34: 748–759.
Bellovin DI, Simpson KJ, Danilov T, Maynard E, Rimm DL, Oettgen P et al. Reciprocal regulation of RhoA and RhoC characterizes the EMT and identifies RhoC as a prognostic marker of colon carcinoma. Oncogene 2006; 25: 6959–6967.
Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell 2008; 135: 510–523.
Sahai E, Marshall CJ . Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 2003; 5: 711–719.
Chen Z, Sun J, Pradines A, Favre G, Adnane J, Sebti SM . Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J Biol Chem 2000; 275: 17974–17978.
Liu AX, Rane N, Liu JP, Prendergast GC . RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol 2001; 21: 6906–6912.
Mazieres J, Tillement V, Allal C, Clanet C, Bobin L, Chen Z et al. Geranylgeranylated, but not farnesylated, RhoB suppresses Ras transformation of NIH-3T3 cells. Exp Cell Res 2005; 304: 354–364.
Adnane J, Muro-Cacho C, Mathews L, Sebti SM, Munoz-Antonia T . Suppression of rho B expression in invasive carcinoma from head and neck cancer patients. Clin Cancer Res 2002; 8: 2225–2232.
Wang S, Yan-Neale Y, Fischer D, Zeremski M, Cai R, Zhu J et al. Histone deacetylase 1 represses the small GTPase RhoB expression in human nonsmall lung carcinoma cell line. Oncogene 2003; 22: 6204–6213.
Sato N, Fukui T, Taniguchi T, Yokoyama T, Kondo M, Nagasaka T et al. RhoB is frequently downregulated in non-small-cell lung cancer and resides in the 2p24 homozygous deletion region of a lung cancer cell line. Int J Cancer 2007; 120: 543–551.
Mazieres J, Antonia T, Daste G, Muro-Cacho C, Berchery D, Tillement V et al. Loss of RhoB expression in human lung cancer progression. Clin Cancer Res 2004; 10: 2742–2750.
Wheeler AP, Ridley AJ . RhoB affects macrophage adhesion, integrin expression and migration. Exp Cell Res 2007; 313: 3505–3516.
Zhou J, Zhu Y, Zhang G, Liu N, Sun L, Liu M et al. A distinct role of RhoB in gastric cancer suppression. Int J Cancer 2011; 128: 1057–1068.
Ho HH, Chang CS, Ho WC, Liao SY, Lin WL, Wang CJ . Gallic acid inhibits gastric cancer cells metastasis and invasive growth via increased expression of RhoB, downregulation of AKT/small GTPase signals and inhibition of NF-kappaB activity. Toxicol Appl Pharmacol 2013; 266: 76–85.
Bousquet E, Mazieres J, Privat M, Rizzati V, Casanova A, Ledoux A et al. Loss of RhoB expression promotes migration and invasion of human bronchial cells via activation of AKT1. Cancer Res 2009; 69: 6092–6099.
Kazerounian S, Gerald D, Huang M, Chin YR, Udayakumar D, Zheng N et al. RhoB differentially controls Akt function in tumor cells and stromal endothelial cells during breast tumorigenesis. Cancer Res 2013; 73: 50–61.
Jackson J, Meisinger J, Patel S, Lim ZC, Vellody K, Metz R et al. Protein phosphatase-2A associates with the cytoskeleton to maintain cell spreading and reduced motility of nonmetastatic Lewis lung carcinoma cells: the loss of this regulatory control in metastatic cells. Invasion Metastasis 1997; 17: 199–209.
Rintoul RC, Sethi T . The role of extracellular matrix in small-cell lung cancer. Lancet Oncol 2001; 2: 437–442.
Carpenter RL, Paw I, Dewhirst MW, Lo HW . Akt phosphorylates and activates HSF-1 independent of heat shock, leading to Slug overexpression and epithelial-mesenchymal transition (EMT) of HER2-overexpressing breast cancer cells. Oncogene 2014; 34: 546–557.
Cordonnier T, Bishop JL, Shiota M, Nip KM, Thaper D, Vahid S et al. Hsp27 regulates EGF/beta-catenin mediated epithelial to mesenchymal transition in prostate cancer. Int J Cancer 2015; 136: E496–E507.
Li Y, Jia L, Ren D, Liu C, Gong Y, Wang N et al. Axl mediates tumor invasion and chemosensitivity through PI3K/Akt signaling pathway and is transcriptionally regulated by slug in breast carcinoma. IUBMB Life 2014; 66: 507–518.
Lin CH, Wang WC, Kao SH . Der p 2 promotes motility of airway epithelial cell attributing to AKT/GSK3beta-associated epithelial-to-mesenchymal transition. Mol Cell Biochem 2014; 395: 135–143.
Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y . Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 2004; 101: 7618–7623.
Delord JP, Quideau S, Rochaix P, Caselles O, Couderc B, Hennebelle I et al. Trastuzumab induced in vivo tissue remodelling associated in vitro with inhibition of the active forms of AKT and PTEN and RhoB induction in an ovarian carcinoma model. Br J Cancer 2010; 103: 61–72.
Lee WJ, Kim DU, Lee MY, Choi KY . Identification of proteins interacting with the catalytic subunit of PP2A by proteomics. Proteomics 2007; 7: 206–214.
Chen J, Parsons S, Brautigan DL . Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J Biol Chem 1994; 269: 7957–7962.
Seshacharyulu P, Pandey P, Datta K, Batra SK . Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett 2013; 335: 9–18.
Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW . Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem 2008; 283: 1882–1892.
Ruvolo PP, Qui YH, Coombes KR, Zhang N, Ruvolo VR, Borthakur G et al. Low expression of PP2A regulatory subunit B55alpha is associated with T308 phosphorylation of AKT and shorter complete remission duration in acute myeloid leukemia patients. Leukemia 2011; 25: 1711–1717.
Rocher G, Letourneux C, Lenormand P, Porteu F . Inhibition of B56-containing protein phosphatase 2As by the early response gene IEX-1 leads to control of Akt activity. J Biol Chem 2007; 282: 5468–5477.
Calvayrac O, Pradines A, Rouquette I, Raymond-Letron I, Bousquet E, Lauwers-Cances V et al. RhoB as a prognostic Bismark in lipidic adenoma. Clin Cancer Res 2014; 20: 6541–6550 In revision.
Gerald D, Adini I, Shechter S, Perruzzi C, Varnau J, Hopkins B et al. RhoB controls coordination of adult angiogenesis and lymphangiogenesis following injury by regulating VEZF1-mediated transcription. Nat Commun 2013; 4: 2824.
Shih JY, Yang PC . The EMT regulator slug and lung carcinogenesis. Carcinogenesis 2011; 32: 1299–1304.
Vega FM, Thomas M, Reymond N, Ridley AJ . The Rho GTPase RhoB regulates cadherin expression and epithelial cell-cell interaction. Cell Commun Signal 2015; 13: 6.
Merikallio H, T TT, Paakko P, Makitaro R, Kaarteenaho R, Lehtonen S et al. Slug is associated with poor survival in squamous cell carcinoma of the lung. Int J Clin Exp Pathol 2014; 7: 5846–5854.
Calvayrac O, Pradines A, Raymond-Letron I, Rouquette I, Bousquet E, Lauwers-Cances V et al. RhoB determines tumor aggressiveness in a murine EGFRL858R-induced adenocarcinoma model and is a potential prognostic biomarker for lepidic lung cancer. Clin Cancer Res 2014; 20: 6541–6550.
Fenouille N, Tichet M, Dufies M, Pottier A, Mogha A, Soo JK et al. The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS One 2012; 7: e40378.
Shih JY, Tsai MF, Chang TH, Chang YL, Yuan A, Yu CJ et al. Transcription repressor slug promotes carcinoma invasion and predicts outcome of patients with lung adenocarcinoma. Clin Cancer Res 2005; 11: 8070–8078.
Barber MA, Welch HC . PI3K and RAC signalling in leukocyte and cancer cell migration. Bull Cancer 2006; 93: E44–E52.
Xu SW, Liu S, Eastwood M, Sonnylal S, Denton CP, Abraham DJ et al. Rac inhibition reverses the phenotype of fibrotic fibroblasts. PLoS One 2009; 4: e7438.
van Rijssel J, Hoogenboezem M, Wester L, Hordijk PL, Van Buul JD . The N-terminal DH-PH domain of Trio induces cell spreading and migration by regulating lamellipodia dynamics in a Rac1-dependent fashion. PLoS One 2012; 7: e29912.
Li Y, Guo Z, Chen H, Dong Z, Pan ZK, Ding H et al. HOXC8-dependent cadherin 11 expression facilitates breast cancer cell migration through Trio and Rac. Genes Cancer 2011; 2: 880–888.
Schmidt S, Debant A . Function and regulation of the Rho guanine nucleotide exchange factor Trio. Small GTPases 2014; 5: e29769.
Fortin SP, Ennis MJ, Schumacher CA, Zylstra-Diegel CR, Williams BO, Ross JT et al. Cdc42 and the guanine nucleotide exchange factors Ect2 and trio mediate Fn14-induced migration and invasion of glioblastoma cells. Mol Cancer Res 2012; 10: 958–968.
DeGeer J, Boudeau J, Schmidt S, Bedford F, Lamarche-Vane N, Debant A . Tyrosine phosphorylation of the Rho guanine nucleotide exchange factor Trio regulates netrin-1/DCC-mediated cortical axon outgrowth. Mol Cell Biol 2013; 33: 739–751.
Liao Y, Hung MC . Physiological regulation of Akt activity and stability. Am J Transl Res 2010; 2: 19–42.
Benefield J, Meisinger J, Petruzzelli GJ, Young MR . Endothelial cell response to human head and neck squamous cell carcinomas involves downregulation of protein phosphatases-1/2A, cytoskeletal depolymerization and increased motility. Invasion Metastasis 1997; 17: 210–220.
Nakada N, Kuroda K, Kawahara E . Protein phosphatase 2A regulatory subunit Bbeta promotes MAP kinase-mediated migration of A431 cells. Cell Physiol Biochem 2005; 15: 19–28.
Sents W, Ivanova E, Lambrecht C, Haesen D, Janssens V . The biogenesis of active protein phosphatase 2A holoenzymes: a tightly regulated process creating phosphatase specificity. FEBS J 2013; 280: 644–661.
Mao KP, Zhang WN, Liang XM, Ma YR . MicroRNA-222 expression and its prognostic potential in non-small cell lung cancer. ScientificWorldJournal 2014 2014. 908326.
Zhang DQ, Zhou CK, Jiang XW, Chen J, Shi BK . Increased expression of miR-222 is associated with poor prognosis in bladder cancer. World J Surg Oncol 2014; 12: 241.
Zhang Y, Ma T, Yang S, Xia M, Xu J, An H et al. High-mobility group A1 proteins enhance the expression of the oncogenic miR-222 in lung cancer cells. Mol Cell Biochem 2011; 357: 363–371.
Mamouni K, Cristini A, Guirouilh-Barbat J, Monferran S, Lemarie A, Faye JC et al. RhoB Promotes gammaH2AX dephosphorylation and DNA double-strand break repair. Mol Cell Biol 2014; 34: 3144–3155.
Acknowledgements
We are grateful to Professor Alain Puisieux (CRCL, Lyon, France) for his helpful comments on the manuscript. We thank Rémi Gence for helping with recombinant proteins. This work was supported by grants from INSERM (Institut National de la Santé et de la Recherche Médicale), Groupe de Recherche Institut Claudius Regaud, the Foundation Recherche et Innovation Thérapeutique en Cancérologie and INCa (Institut National du Cancer) France.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Bousquet, E., Calvayrac, O., Mazières, J. et al. RhoB loss induces Rac1-dependent mesenchymal cell invasion in lung cells through PP2A inhibition. Oncogene 35, 1760–1769 (2016). https://doi.org/10.1038/onc.2015.240
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/onc.2015.240
This article is cited by
-
Nectin-4 is frequently expressed in primary salivary gland cancer and corresponding lymph node metastases and represents an important treatment-related biomarker
Clinical & Experimental Metastasis (2023)
-
Mechanical stress shapes the cancer cell response to neddylation inhibition
Journal of Experimental & Clinical Cancer Research (2022)
-
β-Pix-dependent cellular protrusions propel collective mesoderm migration in the mouse embryo
Nature Communications (2020)
-
TFAP2C increases cell proliferation by downregulating GADD45B and PMAIP1 in non-small cell lung cancer cells
Biological Research (2019)
-
Protein phosphatase 2A (PP2A): a key phosphatase in the progression of chronic obstructive pulmonary disease (COPD) to lung cancer
Respiratory Research (2019)