Abstract
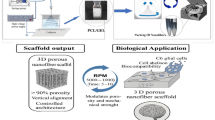
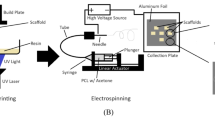
Electrospun gelatin nanofibers are effective tissue engineering scaffolds with high biocompatibility and cell adhesion activity. In gelatin electrospinning, fluorinated alcohols, which are irritants, and acidic organic solvents are used as solvents to prevent gelation. This study established a technique to embed protein reagents into the nanofibers using mild solvents. From 22 mixtures of 50% organic solvent–50% H2O, less denaturing neutral dipolar aprotic solvents (specifically N,N-dimethylacetamide, N,N-dimethylformamide and N-methyl-2-pyrrolidone), were screened to assess their suitability for use in electrospinning of gelatin nanofiber scaffolds by their ability to maintain gelatin in a sol state at room temperature. By selecting the solvents and their concentrations, gelatin nanofibers were electrospun with different structures from a thick, wide, porous nanofibrous structure to a thin, fine, nanofibrous mesh structure. Swiss 3T3 fibroblasts grew well on the gelatin nanofibers. In particular, some cells showed in vivo-like spindle morphologies on the thick porous nanofibers using N,N-dimethylacetamide. Additionally, as a model protein reagent, alkaline phosphatase was embedded in the gelatin nanofibers while maintaining high activity. Considering these results, the gelatin nanofibers in this study are expected to provide effective structural and chemical cues and will be useful for tissue engineering and regenerative medicine.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Evans, M. J. & Kaufman, M. H. Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156 (1981).
Robinton, D. A. & Daley, G. Q. The promise of induced pluripotent stem cells in research and therapy. Nature 481, 295–305 (2012).
Langer, R. & Vacanti, J. P. Tissue engineering. Science 260, 920–926 (1993).
Lu, P., Weaver, V. M. & Werb, Z. The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol. 196, 395–406 (2012).
Bukoreshtliev, N. V., Haase, K. & Pelling, A. E. Mechanical cues in cellular signalling and communication. Cell Tissue Res. 352, 77–94 (2013).
Sill, T. J. & von Recum, H. A. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29, 1989–2006 (2008).
Kai, D., Jin, G., Prabhakaran, M. P. & Ramakrishna, S. Electrospun synthetic and natural nanofibers for regenerative medicine and stem cells. Biotechnol. J 8, 59–72 (2013).
Discher, D. E., Mooney, D. J. & Zandstra, P. W. Growth factors, matrices, and forces combine and control stem cells. Science 324, 1673–1677 (2009).
Schneider, A., Wang, X. Y., Kaplan, D. L., Garlick, J. A. & Egles, C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 5, 2570–2578 (2009).
Ju, J., Yamagata, Y. & Higuchi, T. Thin-film fabrication method for organic light-emitting diodes using electrospray deposition. Adv. Mater. 21, 4343–4347 (2009).
Kim, J. W., Yamagata, Y., Kim, B. J. & Higuchi, T. Direct and dry micro-patterning of nano-particles by electrospray deposition through a micro-stencil mask. J. Micromech. Microeng. 19, 025021–025029 (2009).
Aoki, H., Kaneko, A., Kajita, A., Yamagata, Y., Ike, F. & Kase, H. An on-site serology monitoring system for laboratory mice using a multiplex microfluidic chip fabricated by the electrospray deposition method. J. Chem. Eng. Jpn 45, 528–538 (2012).
Seo, H., Matsumoto, H., Hara, S., Minagawa, M., Tanioka, A., Yako, H., Yamagata, Y., Inoue, K. Preparation of polysaccharide nanofiber fabrics by electrospray deposition: additive effects of poly (ethylene oxide). Polym. J. 37, 391–398 (2005).
Morota, K., Matsumoto, H., Mizukoshi, T., Konosu, Y., Minagawa, M., Tanioka, A., Yamagata, Y. & Inoue, K. Poly(ethylene oxide) thin films produced by electrospray deposition: morphology control and additive effects of alcohols on nanostructure. J. Colloid Interface Sci. 279, 484–492 (2004).
Zhang, Y., Ouyang, H., Lim, C. T., Ramakrishna, S. & Huang, Z. M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 72, 156–165 (2005).
Tsai, S. W., Liou, H. M., Lin, C. J., Kuo, K. L., Hung, Y. S., Weng, R. C. & Hsu, F. Y. MG63 osteoblast-like cells exhibit different behavior when grown on electrospun collagen matrix versus electrospun gelatin matrix. PLoS ONE 7, e31200 (2012).
Choktaweesap, N., Arayanarakul, K., Aht-ong, D., Meechaisue, C., Supaphol, P. Electrospun gelatin fibers: effect of solvent system on morphology and fiber diameters. Polym. J. 39, 622–631 (2007).
Song, J. H., Kim, H. E. & Kim, H. W. Production of electrospun gelatin nanofiber by water-based co-solvent approach. J. Mater. Sci. Mater. Med. 19, 95–102 (2008).
Chen, H. C., Jao, W. C. & Yang, M. C. Characterization of gelatin nanofibers electrospun using ethanol/formic acid/water as a solvent. Polymers Adv. Technol. 20, 98–103 (2009).
Angarano, M., Schulz, S., Fabritius, M., Vogt, R., Steinberg, T., Tomakidi, P., Friedrich, C. & Mülhaupt, R. Layered gradient nonwovens of in situ crosslinked electrospun collagenous nanofibers used as modular scaffold systems for soft tissue regeneration. Adv. Funct. Mater. doi:10.1002/adfm.201202816 (2013).
Zeugolis, D. I., Khew, S. T., Yew, E. S. Y., Ekaputra, A. K., Tong, Y. W., Yung, L. Y. L., Hutmacher, D. W., Sheppard, C. & Raghunath, M. Electro-spinning of pure collagen nano-fibres – Just an expensive way to make gelatin? Biomaterials 29, 2293–2305 (2008).
Zhang, S., Huang, Y., Yang, X., Mei, F., Ma, Q., Chen, G., Ryu, S. & Deng, X. Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regeneration. J. Biomed. Mater. Res. A 90, 671–679 (2009).
Westall, F. C., Rubin, R. & Gospodarowicz, D. Brain-derived fibroblast growth factor: a study of its inactivation. Life Sci. 33, 2425–2429 (1983).
Edelman, E. R., Mathiowitz, E., Langer, R. & Klagsbrun, M. Controlled and modulated release of basic fibroblast growth factor. Biomaterials 12, 619–626 (1991).
Santana, H., Gonzalez, Y., Campana, P. T., Noda, J., Amarantes, O., Itri, R., Beldarrain, A. & Paez, R. Screening for stability and compatibility conditions of recombinant human epidermal growth factor for parenteral formulation: effect of pH, buffers, and excipients. Int. J. Pharm. 452, 52–62 (2013).
Sanwlani, S., Kumar, P. & Bohidar, H. B. Hydration of gelatin molecules in glycerol-water solvent and phase diagram of gelatin organogels. J. Phys. Chem. B 115, 7332–7340 (2011).
Landolt-Börnstein Database, Springer Materials. http://www.springermaterials.com. Accessed 25 July 2013.
Lo, C. C. & Chao, P. M. Replacement of carcinogenic solvent HMPA by DMI in insect sex pheromone synthesis. J. Chem. Ecol. 16, 3245–3253 (1990).
Lide, D. R. (ed.) Properties of Organic Solventss, ver.2.0 (CRC Press: Boca Raton, FL, USA, 1996).
Marcus, Y. The Properties of Solvents (Wiley: Chichester, UK, 1998).
Zhuravlev, V. I., Usacheva, T. M., Lifanova, N. V. & Vydrina, E. P. Dielectric properties of polyhydric alcohols: butanediols. Russ. J. Gen. Chem. 78, 1189–1196 (2008).
Miller, R. G., Bowles, C. Q., Chappelow, C. C. & Eick, J. D. Application of solubility parameter theory to dentin-bonding systems and adhesive strength correlations. J. Biomed. Mater. Res. 41, 237–243 (1998).
Hansen, C. M. Hansen Solubility Parameters: A User's Handbook (CRC Press LLC: Boca Ranton, FL, 1999).
Williams, D. L. & Kuklenz, K. D. A determination of the Hansen solubility parameters of hexanitrostilbene (HNS). Propellants Explos. Pyrotech. 34, 452–457 (2009).
Barton, A. F. M. Handbook of Solubility Parameters and Other Cohesion Parameters, 2nd edn (CRC Press LLC: Boca Ranton, FL, 1991).
Choi, E. Y., Han, T. H., Hong, J., Kim, J. E., Lee, S. H., Kim, H. W. & Kim, S. O. Noncovalent functionalization of graphene with end-functional polymers. J. Mater. Chem. 20, 1907–1912 (2010).
Hayakawa, T., Yoshinari, M., Nitta, K. & Inoue, K. Collagen nanofiber on titanium or partially stabilized zirconia by electrospray deposition. J. Hard Tissue Biol 19, 5–12 (2010).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Jayakrishnan, A. & Jameela, S. R. Glutaraldehyde as a fixative in bioprostheses and drug delivery matrices. Biomaterials 17, 471–484 (1996).
Passey, S., Pellegrin, S. & Mellor, H. Scanning electron microscopy of cell surface morphology. Curr. Protoc. Cell Biol. Chapter 4: Unit 4.17 (2007).
Fernley, H. in The Enzymes 3rd edn Vol 4: ed Boyer P. D. pp. 417–447 (Academic Press: New York, NY, USA, 1971).
Wu, J., Chiu, S., Pearce, E. M. & Kwei, T. K. Effects of phenolic compounds on gelation behavior of gelatin gels. J. Polym. Sci. A Polym. Chem. 39, 224–231 (2001).
Miyawaki, O., Norimatsu, Y., Kumagai, H., Irimoto, Y., Kumagai, H. & Sakurai, H. Effect of water potential on sol-gel transition and intermolecular interaction of gelatin near the transition temperature. Biopolymers 70, 482–491 (2003).
Yamada, K. M. & Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 130, 601–610 (2007).
Friedl, P. & Alexander, S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell 147, 992–1009 (2011).
Cohen, D. E. & Melton, D. Turning straw into gold: directing cell fate for regenerative medicine. Nat. Rev. Genet. 12, 243–252 (2011).
Gkioni, K., Leeuwenburgh, S. C., Douglas, T. E., Mikos, A. G. & Jansen, J. A. Mineralization of hydrogels for bone regeneration. Tissue Eng. B Rev. 16, 577–585 (2010).
Umberger, J. Q. Solution and gelation of gelatin as related to solvent structure. Photogr. Sci. Eng. 11, 385–391 (1967).
Parker, A. J. The effects of solvation on the properties of anions in dipolar aprotic solvents. Q. Rev. Chem. Soc 16, 163–187 (1962).
Ewell, R. H., Harrison, J. M. & Berg, L. Azeotropic distillation. Ind. Eng. Chem 36, 871–875 (1944).
ChemIDplus Advanced. US National Library of Medicine (2014). http://chem.sis.nlm.nih.gov/chemidplus/. Accessed 15 May 2014.
Halocarbon, Hexafluoroisopropanol (HFIP) MSDS (2015). http://www.halocarbon.com/. Accessed 15 May 2014.
Sanghvi, R., Narazaki, R., Machatha, S. G. & Yalkowsky, S. H. Solubility improvement of drugs using N-methyl pyrrolidone. AAPS Pharm. Sci. Tech. 9, 366–376 (2008).
Jouyban, A., Fakhree, M. A. & Shayanfar, A. Review of pharmaceutical applications of N-methyl-2-pyrrolidone. J. Pharm. Pharmaceut. Sci. 13, 524–535 (2010).
Ligocka, D., Lison, D. & Haufroid, V. Contribution of CYP2E1 to N-methyl-2-pyrrolidone metabolism. Arch. Toxicol. 77, 261–266 (2003).
Schwartz, M. A. & Chen, C. S. Cell biology. Deconstructing dimensionality. Science 339, 402–404 (2013).
Christopherson, G. T., Song, H. & Mao, H. Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials 30, 556–564 (2009).
Luria, E. A., Owen, M. E., Friedenstein, A. J., Morris, J. F. & Kuznetsow, S. A. Bone formation in organ cultures of bone marrow. Cell Tissue Res. 248, 449–454 (1987).
Doughty, M. J. Assessment of collagen fibril spacing in relation to selected region of interest (ROI) on electron micrographs–application to the mammalian corneal stroma. Microsc. Res. Tech. 75, 474–483 (2012).
Franchi, M., Trire, A., Quaranta, M., Orsini, E. & Ottani, V. Collagen structure of tendon relates to function. ScientificWorldJournal 7, 404–420 (2007).
Ji, W., Sun, Y., Yang, F., van, J. J., Fan, M., Chen, Z. & Jansen, J. A. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharm. Res. 28, 1259–1272 (2011).
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 23510139 and RIKEN. We thank Mr D Inoue at the Materials Characterization Support Unit at the RIKEN Center for Emergent Matter Science for his technical SEM support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Rights and permissions
About this article
Cite this article
Aoki, H., Miyoshi, H. & Yamagata, Y. Electrospinning of gelatin nanofiber scaffolds with mild neutral cosolvents for use in tissue engineering. Polym J 47, 267–277 (2015). https://doi.org/10.1038/pj.2014.94
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pj.2014.94
This article is cited by
-
Electrospun Gelatin Nanofibres—Fabrication, Cross-linking and Biomedical Applications: A Review
Biomedical Materials & Devices (2023)
-
Fabrication of gelatin nanofiber webs via centrifugal spinning for N95 respiratory filters
Bulletin of Materials Science (2022)