Abstract
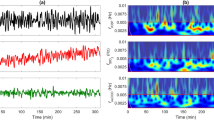
In preterm infants intraventricular hemorrhage occurs predominantly within the perinatal period, which may be due to a “lost autoregulation” of cerebral blood flow (CBF). In this study, perinatal autoregulation dynamics were investigated in high risk preterm infants by cross-spectral analysis (CSA), which is a statistical tool in the analysis of time series. In 15 ventilated preterm infants of 25-32 gestational weeks, a total number of 30 records were made between 24 and 96 h of life. Doppler-derived CBF velocity (CBFv), used as a quantitative measure for CBF, and direct mean arterial blood pressure (MABP) were measured continuously for 10 min. The spectral power of low frequency (LF, 0.02-0.2 Hz) oscillations in CBFv and MABP was quantified by spectral analysis. From the results of CSA, a LF phaseshift between the CBFv and MABP LF oscillations was calculated in each record. Within the study group, the LF spectral power of CBFv and MABP was initially low and increased significantly until 96 h of life. The LF phase-shift was about 0 ° at 24 h and increased significantly to 55 ° at 96 h of life. The initially low LF spectral power of CBFv and MABP may indicate a perinatal depression of autonomic nervous centers, which are thought to control LF oscillations of vital parameters. In the light of a high pass filter model for autoregulation, the initially low LF phase-shift may indicate an initially impaired autoregulation, which supports the “lost autoregulation” hypothesis.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- IVH:
-
intraventricular hemorrhage
- gw:
-
gestational weeks
- CBF(v):
-
cerebral blood flow (velocity)
- (M)ABP:
-
(mean) arterial blood pressure
- CSA:
-
cross-spectral analysis
- LF:
-
low frequency band (0.02-0.2 Hz)
- SPLF:
-
total spectral power within the LF band
- MABP-SPLF:
-
spectral power of MABP
- CBFv-SPLF:
-
LF spectral power of CBFv
- phase-shiftLF:
-
LF phase-shift between CBFv and MABP
- coherenceLF:
-
LF coherence between CBFv and MABP
- log-center-freqLF:
-
LF logarithmic center frequency
- T1/2:
-
half-maximal CBFv response time
References
Lou HC 1988 The “lost autoregulation hypothesis” and brain lesions in the newborn-an update. Brain Dev 10: 143–146.
Shortland DB, Levene M, Archer N, Shaw D, Evans D 1990 Cerebral blood flow velocity recordings and the prediction of intracranial haemorrhage and ischemia. J Perinat Med 18: 411–417.
Altman DI, Volpe JJ 1987 Cerebral blood flow in the newborn infant: measurement and role in the pathogenesis of periventricular and intraventricular hemorrhage. Adv Pediatr 34: 111–138.
Dykes FD, Ahmann PA, Baldzer K, Carrigan TA, Kitney R, Giddens DP 1986 Breath amplitude modulation of heart rate variability in normal full term neonates. Pediatr Res 20: 301–308.
Malliani A, Pagani M, Lombardi F, Cerutti S 1991 Cardiovascular neural regulation explored in the frequency domain. Circulation 84: 482–492.
Chatow U, Davidson S, Reichman BL, Akselrod S 1995 Development and maturation of the autonomic nervous system in premature and full-term infants using spectral analysis of heart rate fluctuations. Pediatr Res 37: 294–302.
Giller CA, Bowmann G, Dyer H, Mootz L, Krippner W 1993 Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 32: 737–742.
Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR 1994 Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke 25: 793–797.
Haaland K, Karlsson B, Skovlund E, Thoresen M 1994 Simultaneous measurements of cerebral circulation with electromagnetic flowmetry and Doppler ultrasound velocity in the newborn pig. Pediatr Res 36: 601–606.
Michel E, Zernikow B, Steck J, Kohlmann G, von Siebenthal K, Hirano S, Fock A, Casaer P, Jorch G 1994 Cyclic variation pattern of cerebral blood flow and postconceptional age. Eur J Pediatr 153: 751–755.
Anthony MY, Evans DH, Levene MI 1991 Cyclical variations in cerebral blood flow velocity. Arch Dis Child 66: 12–16.
Coughtrey H, Rennie JM, Evans DH 1992 Postnatal evolution of slow variability in cerebral blood flow velocity. Arch Dis Child 67: 412–415.
Paulson OB, Strandgard S, Edvinsson L 1990 Cerebral autoregulation. Cerebrovasc Brain Metab Rev 2: 161–192.
Tiecks FP, Lam AM, Aaslid R, Newell DW 1995 Comparison of static and dynamic cerebral autoregulation measurements. Stroke 26: 1014–1019.
Chatfield C 1989 The Analysis of Time Series: An Introduction, 4th ed. Chapman & Hall, London, pp 105–180.
Aaslid R, Lindegard KF, Sorteberg W, Nornes H 1989 Cerebral autoregulation dynamics in humans. Stroke 20: 45–52.
Birch AA, Dirnhuber MJ, Hartley-Davies R, Iannotti F, Neil-Dwyer G 1995 Assessment of autoregulation by means of periodic changes in blood pressure. Stroke 26: 834–837.
Diehl RR, Linden D, Lucke D, Berlit P 1995 Phase relationship between cerebral blood flow velocity and blood pressure. A clinical test of autoregulation. Stroke 26: 1801–1804.
Panerai RB, Kelsall AWR, Rennie JM, Evans DH 1995 Cerebral autoregulation dynamics in premature newborns. Stroke 26: 74–80.
Michel E, Zernikow B, von Twickel J, Hillebrand S, Jorch G 1995 Critical closing pressure in preterm neonates: towards a comprehensive model of cerebral autoregulation. Neurol Res 17: 149–155.
Michel E, Zernikow B 1994 Sample! Low cost hard- and software for 16-channel pressure monitoring. In: Mutz NJ, Koller W, Benzer H (eds) 7th European Congress on Intensive Care Medicine. Monduzzi Editore, Bologna, Italy, pp 1055–1058.
Michel E, Zernikow B, Rabe H, Jorch G 1993 Adaptive multipurpose probe fixation device for use on newborns. Ultrasound Med Biol 19: 581–586.
Zernikow B, Michel E, Kohlmann G, Steck J, Schmitt RM, Jorch G 1994 Cerebral autoregulation of preterm neonates-a non-linear control system? Arch Dis Child 70:F166–F173.
Rabe H, Grohs B, Schmidt RM, Schloo R, Bömelburg T, Jorch G 1990 Acoustic power measurements of Doppler ultrasound devices used for perinatal and infant examinations. Pediatr Radiol 20: 277–281.
Shimada SG, Marsh DJ 1979 Oscillations in mean arterial blood pressure in conscious dogs. Circ Res 44: 692–700.
Hamming RW 1983 Digital Filters. Prentice-Hall, Englewood Cliffs, NJ, pp 121–160.
Shott S 1990 Statistics for Health Professionals. WB Saunders, Philadelphia, pp 269–312.
Bignall S, Bailey PC, Rivers RPA, Lissauer TJ 1988 Quantification of cardiovascular instability in premature infants using spectral analysis of waveforms. Pediatr Res 23: 398–401.
Diehl RR, Diehl B, Sitzer M, Hennerici M 1991 Spontaneous oscillations in cerebral blood flow velocity in normal humans and in patients with carotid artery disease. Neurosci Lett 127: 5–8.
Menke J, Michel E, Rabe H, Bresser BW, Grohs B, Schmitt RM, Jorch G 1993 Simultaneous influence of blood pressure, Pco2, and Po2 on cerebral blood flow velocity in preterm infants of less than 33 weeks gestation. Pediatr Res 34: 173–177.
Rake H 1991 Regelungstechnik A und Ergänzungen (Regelungstechnik B). Institut für Regelungstechnik, Rheinisch-Westfälische Technische Hochschule Aachen, Aachen, pp 4/1–4/46
Heinbockel B Voβ HJ 1989 Alles geregelt-Reglersimulationsprogramm in Turbo Pascal 4/5. c't August 1989, Verlag Heinz Heise, Hannover, pp 140–160
Acknowledgements
The authors thank the parents and the nurses of the intensive care unit for their support.
Author information
Authors and Affiliations
Additional information
Supported in part by a grant of the Deutsche Forschungsgemeinschaft (Jo 156-2).
The raw data are part of the theses of J.v.T. and S.H.
Appendices
Appendix A
Description of LF spectral parameters. The following symbols are used: Equation

Calculation of LF spectral parameters Equation

Appendix B
Simple high pass filter model for dynamic cerebral autoregulation. In the time domain the general equation of a simple high pass filter system is given by (31): Equation

In control engineering theory this first-order differential equation describes a so-called DT1-element (31), which is a combination of a differential (D) and one (1) temporal (T) element and acts as a high pass filter (15, 17, 18). A high pass filter dampens especially LF MABP oscillations. High frequency MABP oscillations pass the high pass filter and lead to corresponding disturbances in CBF. The effect of step, sinusoidal, or more complex MABP changes on CBF can be visualized by computer simulation (32). Step and sinusoidal MABP changes and their specific CBF responses (Fig. 4) are only special cases of this generalized model. The general equation of the DT1-element can then be specified:
1) Step MABP increase (Fig. 4, A and C): Equation

Rights and permissions
About this article
Cite this article
Menke, J., Michel, E., Hillebrand, S. et al. Cross-Spectral Analysis of Cerebral Autoregulation Dynamics in High Risk Preterm Infants during the Perinatal Period. Pediatr Res 42, 690–699 (1997). https://doi.org/10.1203/00006450-199711000-00023
Received:
Accepted:
Issue date:
DOI: https://doi.org/10.1203/00006450-199711000-00023
This article is cited by
-
Ultrasound imaging of preterm brain injury: fundamentals and updates
Pediatric Radiology (2022)
-
Cerebral oxygen saturation and autoregulation during hypotension in extremely preterm infants
Pediatric Research (2021)
-
Reduction of brain volumes after neonatal cardiopulmonary bypass surgery in single-ventricle congenital heart disease before Fontan completion
Pediatric Research (2018)
-
The Instrumented Fetal Sheep as a Model of Cerebral White Matter Injury in the Premature Infant
Neurotherapeutics (2012)
-
Cerebral Blood Flow Heterogeneity in Preterm Sheep: Lack of Physiologic Support for Vascular Boundary Zones in Fetal Cerebral White Matter
Journal of Cerebral Blood Flow & Metabolism (2008)