Abstract
Background:
Intrauterine growth restriction (IUGR) is an important risk factor for cardiovascular disease. Previous studies revealed altered myocardial matrix composition after IUGR. We hypothesized that IUGR is accompanied by compromised myocardial performance independently from arterial hypertension.
Methods:
IUGR was induced in Wistar rats by maternal protein restriction, and hearts of male offspring were studied using echocardiography, immunohistochemistry, real-time PCR, and western blot analysis.
Results:
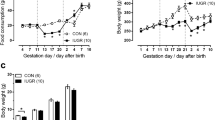
At day 70 of life, in the absence of arterial hypertension (mean arterial blood pressure: 101.3 ± 7.1 mmHg in IUGR vs. 105.3 ± 4.6 mmHg in controls, not significant (NS)), echocardiography showed a reduced contractility (ejection fraction: 65.4 ± 1.8% in IUGR vs. 82.2 ± 1.5% in controls, P < 0.001) of a more distensible myocardium in IUGR rats. Altered expression patterns of myosin chains and titin isoforms and increased expression levels of atrial natriuretic peptide, Na/K-ATPase, and β-adrenergic receptor 1 were detected. A higher number of cardiac fibroblasts and vascular cross-sections were observed in IUGR rats, accompanied by elevated expression of hypoxia inducible factor 1 target genes, such as vascular endothelial growth factor and its receptors.
Conclusion:
We observed a blood pressure–independent impairment of myocardial function after IUGR, which possibly favors cardiovascular disease later in life. Some IUGR-induced myocardial changes (e.g., sarcomeric components) may partly explain the compromised cardiac performance, whereas others (e.g., elevated vascular supply) reflect compensatory mechanisms.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Barker DJ . In utero programming of cardiovascular disease. Theriogenology 2000;53:555–74.
McMillen IC, Robinson JS . Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 2005;85:571–633.
Barker DJ . In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–28.
Corstius HB, Zimanyi MA, Maka N, et al. Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr Res 2005;57:796–800.
Tintu A, Rouwet E, Verlohren S, et al. Hypoxia induces dilated cardiomyopathy in the chick embryo: mechanism, intervention, and long-term consequences. PLoS One 2009;4:e5155.
Crispi F, Bijnens B, Figueras F, et al. Fetal growth restriction results in remodeled and less efficient hearts in children. Circulation 2010;121:2427–36.
Lim K, Lombardo P, Schneider-Kolsky M, Black MJ . Intrauterine growth restriction coupled with hyperglycemia: effects on cardiac structure in adult rats. Pediatr Res 2012;72:344–51.
Cheema KK, Dent MR, Saini HK, Aroutiounova N, Tappia PS . Prenatal exposure to maternal undernutrition induces adult cardiac dysfunction. Br J Nutr 2005;93:471–7.
Vehaskari VM, Woods LL . Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol 2005;16:2545–56.
Menendez-Castro C, Fahlbusch F, Cordasic N, et al. Early and late postnatal myocardial and vascular changes in a protein restriction rat model of intrauterine growth restriction. PLoS One 2011;6:e20369.
Andreollo NA, Santos EF, Araújo MR, Lopes LR . Rat’s age versus human’s age: what is the relationship? Arq Bras Cir Dig 2012;25:49–51.
Battista MC, Calvo E, Chorvatova A, Comte B, Corbeil J, Brochu M . Intra-uterine growth restriction and the programming of left ventricular remodelling in female rats. J Physiol 2005;565(Pt 1):197–205.
Langley-Evans SC, Welham SJ, Jackson AA . Fetal exposure to a maternal low protein diet impairs nephrogenesis and promotes hypertension in the rat. Life Sci 1999;64:965–74.
Hoppe CC, Evans RG, Moritz KM, et al. Combined prenatal and postnatal protein restriction influences adult kidney structure, function, and arterial pressure. Am J Physiol Regul Integr Comp Physiol 2007;292:R462–9.
Zohdi V, Sutherland MR, Lim K, Gubhaju L, Zimanyi MA, Black MJ . Low birth weight due to intrauterine growth restriction and/or preterm birth: effects on nephron number and long-term renal health. Int J Nephrol 2012;2012:136942.
Zohdi V, Jane Black M, Pearson JT . Elevated vascular resistance and afterload reduce the cardiac output response to dobutamine in early growth-restricted rats in adulthood. Br J Nutr 2011;106:1374–82.
Gupta M, Zak R . Reversibility of load-induced changes in myosin heavy chain gene expression. Am J Physiol 1992;262(3 Pt 2):R346–9.
Abraham WT, Gilbert EM, Lowes BD, et al. Coordinate changes in Myosin heavy chain isoform gene expression are selectively associated with alterations in dilated cardiomyopathy phenotype. Mol Med 2002;8:750–60.
Sugiura S, Kobayakawa N, Fujita H, et al. Comparison of unitary displacements and forces between 2 cardiac myosin isoforms by the optical trap technique: molecular basis for cardiac adaptation. Circ Res 1998;82:1029–34.
James J, Martin L, Krenz M, et al. Forced expression of alpha-myosin heavy chain in the rabbit ventricle results in cardioprotection under cardiomyopathic conditions. Circulation 2005;111:2339–46.
Cazorla O, Freiburg A, Helmes M, et al. Differential expression of cardiac titin isoforms and modulation of cellular stiffness. Circ Res 2000;86:59–67.
Neagoe C, Opitz CA, Makarenko I, Linke WA . Gigantic variety: expression patterns of titin isoforms in striated muscles and consequences for myofibrillar passive stiffness. J Muscle Res Cell Motil 2003;24:175–89.
Makarenko I, Opitz CA, Leake MC, et al. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res 2004;95:708–16.
Fan D, Takawale A, Lee J, Kassiri Z . Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis Tissue Repair 2012;5:15.
Izumo S, Nadal-Ginard B, Mahdavi V . Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci USA 1988;85:339–43.
Zeller R, Bloch KD, Williams BS, Arceci RJ, Seidman CE . Localized expression of the atrial natriuretic factor gene during cardiac embryogenesis. Genes Dev 1987;1:693–8.
Adachi S, Ito H, Ohta Y, et al. Distribution of mRNAs for natriuretic peptides in RV hypertrophy after pulmonary arterial banding. Am J Physiol 1995;268(1 Pt 2):H162–9.
Bahlmann F, Krummenauer F, Spahn S, Gallinat R, Kampmann C . Natriuretic peptide levels in intrauterine growth-restricted fetuses with absent and reversed end-diastolic flow of the umbilical artery in relation to ductus venosus flow velocities. J Perinat Med 2011;39:529–37.
Klinger JR, Pietras L, Warburton R, Hill NS . Reduced oxygen tension increases atrial natriuretic peptide release from atrial cardiocytes. Exp Biol Med (Maywood) 2001;226:847–53.
Port JD, Bristow MR . Altered beta-adrenergic receptor gene regulation and signaling in chronic heart failure. J Mol Cell Cardiol 2001;33:887–905.
Xu KY, Takimoto E, Fedarko NS . Activation of (Na+ + K+)-ATPase induces positive inotropy in intact mouse heart in vivo. Biochem Biophys Res Commun 2006;349:582–7.
Jeremy RW, Fletcher PJ, Thompson J . Coronary pressure-flow relations in hypertensive left ventricular hypertrophy. Comparison of intact autoregulation with physiological and pharmacological vasodilation in the dog. Circ Res 1989;65:224–36.
Tomanek RJ . Response of the coronary vasculature to myocardial hypertrophy. J Am Coll Cardiol 1990;15:528–33.
Leychenko A, Konorev E, Jijiwa M, Matter ML . Stretch-induced hypertrophy activates NFkB-mediated VEGF secretion in adult cardiomyocytes. PLoS One 2011;6:e29055.
Arola A, Tuominen J, Ruuskanen O, Jokinen E . Idiopathic dilated cardiomyopathy in children: prognostic indicators and outcome. Pediatrics 1998;101(3 Pt 1):369–76.
Plank C, Ostreicher I, Hartner A, et al. Intrauterine growth retardation aggravates the course of acute mesangioproliferative glomerulonephritis in the rat. Kidney Int 2006;70:1974–82.
Teekakirikul P, Eminaga S, Toka O, et al. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J Clin Invest 2010;120:3520–9.
Haas CS, Amann K, Schittny J, Blaser B, Müller U, Hartner A . Glomerular and renal vascular structural changes in alpha8 integrin-deficient mice. J Am Soc Nephrol 2003;14:2288–96.
Hartner A, Cordasic N, Rascher W, Hilgers KF . Deletion of the alpha8 integrin gene does not protect mice from myocardial fibrosis in DOCA hypertension. Am J Hypertens 2009;22:92–9.
Acknowledgements
We gratefully acknowledge the expert technical assistance of Ilona Winterfeld and Miroslava Kupraszewicz-Hutzler.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Menendez-Castro, C., Toka, O., Fahlbusch, F. et al. Impaired myocardial performance in a normotensive rat model of intrauterine growth restriction. Pediatr Res 75, 697–706 (2014). https://doi.org/10.1038/pr.2014.27
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2014.27
This article is cited by
-
Cardiovascular Dysfunction in Intrauterine Growth Restriction
Current Hypertension Reports (2022)
-
Echocardiographic assessment of fetal cardiac function in the uterine artery ligation rat model of IUGR
Pediatric Research (2021)
-
RNA sequencing reveals induction of specific renal inflammatory pathways in a rat model of malignant hypertension
Journal of Molecular Medicine (2021)
-
Intrauterine growth restriction - impact on cardiovascular diseases later in life
Molecular and Cellular Pediatrics (2018)
-
Fetal origins of adult cardiac disease: a novel approach to prevent fetal growth restriction induced cardiac dysfunction using insulin like growth factor
Pediatric Research (2017)