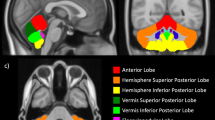
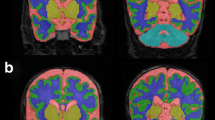
Abstract
Background and Objective
To investigate the relation of early brain activity with structural (growth of the cortex and cerebellum) and white matter microstructural brain development.
Methods
A total of 33 preterm neonates (gestational age 26±1 weeks) without major brain abnormalities were continuously monitored with electroencephalography during the first 48 h of life. Rate of spontaneous activity transients per minute (SAT rate) and inter-SAT interval (ISI) in seconds per minute were calculated. Infants underwent brain magnetic resonance imaging ∼30 (mean 30.5; min: 29.3–max: 32.0) and 40 (41.1; 40.0–41.8) weeks of postmenstrual age. Increase in cerebellar volume, cortical gray matter volume, gyrification index, fractional anisotropy (FA) of posterior limb of the internal capsule, and corpus callosum (CC) were measured.
Results
SAT rate was positively associated with cerebellar growth (P=0.01), volumetric growth of the cortex (P=0.027), increase in gyrification (P=0.043), and increase in FA of the CC (P=0.037). ISI was negatively associated with cerebellar growth (P=0.002).
Conclusions
Increased early brain activity is associated with cerebellar and cortical growth structures with rapid development during preterm life. Higher brain activity is related to FA microstructural changes in the CC, a region responsible for interhemispheric connections. This study underlines the importance of brain activity for microstructural brain development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Vanhatalo S, Kaila K . Development of neonatal EEG activity: from phenomenology to physiology. Semin Fetal Neonatal Med 2006;11:471–478.
Griesmaier E, Enot DP, Bachmann M et al. Systematic characterization of amplitude-integrated EEG signals for monitoring the preterm brain. Pediatr Res 2013;73:226–235.
Toet MC, van Rooij LG, de Vries LS . The use of amplitude integrated electroencephalography for assessing neonatal neurologic injury. Clin Perinatol 2008;35:665–678 v.
Hellstrom-Westas L, Rosen I . Continuous brain-function monitoring: state of the art in clinical practice. Semin Fetal Neonatal Med 2006;11:503–511.
Blumberg MS, Marques HG, Iida F . Twitching in sensorimotor development from sleeping rats to robots. Curr Biol 2013;23:R532–R537.
Colonnese MT, Khazipov R . “Slow activity transients”in infant rat visual cortex: a spreading synchronous oscillation patterned by retinal waves. J Neurosci 2010;30:4325–4337.
Colonnese Mt, Kaminska A, Minlebaev M et al. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron 2010;67:480–498.
Benders MJ, Palmu K, Menache C et al. Early brain activity relates to subsequent brain growth in premature infants. Cereb Cortex 2015;25:3014–3024.
Pandit AS, Ball G, Edwards AD, Counsell SJ . Diffusion magnetic resonance imaging in preterm brain injury. Neuroradiology 2013;55 (Suppl 2): 65–95.
Counsell SJ, Edwards AD, Chew AT et al. Specific relations between neurodevelopmental abilities and white matter microstructure in children born preterm. Brain 2008;131 (Part 12): 3201–3208.
Volpe JJ . Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 2009;8:110–124.
Limperopoulos C, Chilingaryan G, Sullivan N, Guizard N, Robertson RL, du Plessis AJ . Injury to the premature cerebellum: outcome is related to remote cortical development. Cereb Cortex 2014;24:728–736.
Norman E, Wikstrom S, Rosen I, Fellman V, Hellstrom-Westas L . Premedication for intubation with morphine causes prolonged depression of electrocortical background activity in preterm infants. Pediatr Res 2013;73:87–94.
Visser GH, Eilers PH, Elferink-Stinkens PM, Merkus HM, Wit JM . New Dutch reference curves for birthweight by gestational age. Early Hum Dev 2009;85:737–744.
Papile LA, Burstein J, Burstein R, Koffler H . Incidence and evolution of subependimal and intraventricular hemorrhage: a study of infants with birth weight less than 1500 gm. J Pediatr 1978;92:529–534.
Brouwer AJ, van Stam C, Uniken Venema M, Koopman C, Groenendaal F, de Vries LS . Cognitive and neurological outcome at the age of 5–8 years of preterm infants with post-hemorrhagic ventricular dilatation requiring neurosurgical intervention. Neonatology 2012;101:210–216.
de Vries LS, Eken P, Dubowitz LM . The spectrum of leukomalacia using cranial ultrasound. Behav Brain Res 1992;49:1–6.
Kidokoro H, Neil JJ, Inder TE . New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol 2013;34:2208–2214.
Wikstrom S, Pupp IH, Rosen I et al. Early single-channel aEEG/EEG predicts outcome in very preterm infants. Acta Paediatr 2012;101:719–726.
Makropoulos A, Gousias IS, Ledig C et al. Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans Med Imag 2014;33:1818–1831.
Moeskops P, Benders MJ, Chit SM et al. Automatic segmentation of MR brain images of preterm infants using supervised classification. Neuroimage 2015;118:628–641.
Moeskops P, Benders MJ, Kersbergen KJ et al. Development of cortical morphology evaluated with longitudinal MR brain images of preterm infants. PLoS ONE 2015;10:e0131552.
Leemans A, Jones DK . The B-matrix must be rotated when correcting for subject motion in DTI data. Magn Reson Med 2009;61:1336–1349.
Tax CM, Otte WM, Viergever MA, Dijkhuizen RM, Leemans A . REKINDLE: robust extraction of kurtosis INDices with linear estimation. Magn Reson Med 2015;73:794–808.
Oishi K, Mori S, Dnohue PK et al. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage 2011;56:8–20.
Kersbergen KJ, Makropoulos A, Aljabar P et al. Longitudinal regional brain development and clinical risk factors in extremely preterm infants. J Pediatr 2016;178:e6.
Vanhatalo S, Palva JM, Andersson S, Rivera C, Voipio J, Kaila K . Slow endogenous activity transients and developmental expression of K+-Cl-cotransporter 2 in the immature human cortex. Eur J Neurosci 2005;22:2799–2804.
Smyser CD, Inder TE, Shimony JS et al. Longitudinal analysis of neural network development in preterm infants. Cereb Cortex 2010;20:2852–2862.
Natalucci G, Leuchter RH, Bucher HU et al. Functional brain maturation assessed during early life correlates with anatomical brain maturation at term-equivalent age in preterm infants. Pediatr Res 2013;74:68–74.
Innocenti GM, Price DJ . Exuberance in the development of cortical networks. Nat Rev Neurosci 2005;6:955–965.
Kostovic I, Judas M . The development of the subplate and thalamocortical connections in the human foetal brain. Acta Paediatr 2010;99:1119–1127.
Scher M Electroencephalography of the newborn: normal and abnormal features In: Niedermeyer E, Lopez da Silva F eds. Electroencephalography: basic principles, clinical applications, and related fields. Boston, Massachussetts: Lippincott–Williams and Wilkins, 2004: 937–990.
Biagioni E, Frisone MF, Laroche S et al. Maturation of cerebral electrical activity and development of cortical folding in young very preterm infants. Clin Neurophysiol 2007;118:53–59.
Ranasinge S, Or G, Wang EY et al. Reduced cortical activity impairs development and plasticity after neonatal hypoxia ischemia. J Neurosci 2015;35:11946–11959.
Limperopoulos C, Bassan H, Gauvreau K et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics 2007;120:584–593.
Messerschmidt A, Brugger PC, Boltshauser E et al. Disruption of cerebellar development: potential complication of extreme prematurity. Am J Neuroradiol 2005;26:1659–1667.
Dubois J, Benders M, Borradori-Tolsa C et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain 2008;131 (Part 8): 2028–2041.
Eaton DG, Wertheim D, Oozeer R, Dubowitz LM, Dubowitz V . Reversible changes in cerebral activity associated with acidosis in preterm neonates. Acta Paediatr 1994;83:486–492.
Hanganu-Opatz IL . Between molecules and experience: role of early patterns of coordinated activity for the development of cortical maps and sensory abilities. Brain Res Rev 2010;64:160–176.
Colonnese M, Khazipov R . Spontaneous activity in developing sensory circuits: implications for resting state fMRI. Neuroimage 2012;62:2212–2221.
Nimmervoll B, White R, Yang JW et al. LPS-induced microglial secretion of TNFalpha increases activity-dependent neuronal apoptosis in the neonatal cerebral cortex. Cereb Cortex 2013;23:1742–1755.
Tolner EA, Sheikh A, Yukin AY, Kaila K, Kanold PO . Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J Neurosci 2012;32:692–702.
Petanjek Z, Judas M, Simic G et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA 2011;108:13281–13286.
van Kooij BJ, de Vries LS, Ball G et al. Neonatal tract-based spatial statistics findings and outcome in preterm infants. Am J Neuroradio 2012;33:188–194.
Ment LR, Hirtz D, Huppi PS . Imaging biomarkers of outcome in the developing preterm brain. Lancet Neurol 2009;8:1042–1055.
Schmahmann JD, Pandya DN, Wang R et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 2007;130 (Part 3): 630–653.
Kersbergen KJ, Leemans A, Groenendaal F et al. Microstructural brain development between 30 and 40 weeks corrected age in a longitudinal cohort of extremely preterm infants. Neuroimage 2014;103:214–224.
Acknowledgements
This work includes infants participating in the Neobrain study (LSHM-CT-2006-036534). The first draft of the manuscript has been written by M.L.T. and N.H.P.C. Part of this study was presented as an abstract at the 5th Congress of the European Academy of Paediatric Societies (EAPS) 17–21 October 2014, Barcelona (Spain), PS47.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
The first two authors share first authorship.
Statement of Financial Support
The research of A.L. is supported by VIDI Grant 639.072.411 from the Netherlands Organization for Scientific Research (NWO).
Supplementary material is linked to the online version of the paper at
Supplementary information
Rights and permissions
About this article
Cite this article
Tataranno, M., Claessens, N., Moeskops, P. et al. Changes in brain morphology and microstructure in relation to early brain activity in extremely preterm infants. Pediatr Res 83, 834–842 (2018). https://doi.org/10.1038/pr.2017.314
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.314
This article is cited by
-
Cerebellar Development and the Burden of Prematurity
The Cerebellum (2025)
-
Sleep as a driver of pre- and postnatal brain development
Pediatric Research (2024)
-
Sensory-based interventions in the NICU: systematic review of effects on preterm brain development
Pediatric Research (2022)
-
Relationship Between Early Functional and Structural Brain Developments and Brain Injury in Preterm Infants
The Cerebellum (2021)