Abstract
Background
Pregnant women at a high risk of preterm delivery receive glucocorticoids to accelerate fetal lung maturation and surfactant synthesis. However, the effect of antenatal steroids on the developing diaphragm remains unclear. We hypothesized that maternal betamethasone impairs the fetal diaphragm, and the magnitude of the detrimental effect increases with longer duration of exposure. We aimed to determine how different durations of fetal exposure to maternal betamethasone treatment influence the fetal diaphragm at the functional and molecular levels.
Methods
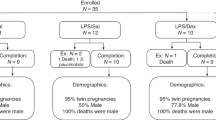
Date-mated merino ewes received intramuscular injections of saline (control) or two doses of betamethasone (5.7 mg) at an interval of 24 h commencing either 2 or 14 days before delivery. Preterm lambs were killed after cesarean delivery at 121-day gestational age. In vitro contractile measurements were performed on the right hemidiaphragm, whereas molecular/cellular analyses used the left costal diaphragm.
Results
Different durations of fetal exposure to maternal betamethasone had no consistent effect on the protein metabolic pathway, expression of glucocorticoid receptor and its target genes, cellular oxidative status, or contractile properties of the fetal lamb diaphragm.
Conclusion
These data suggest that the potential benefits of betamethasone exposure on preterm respiratory function are not compromised by impaired diaphragm function after low-dose maternal intramuscular glucocorticoid exposure.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Leviton LC, Goldenberg RL, Baker CS et al, Methods to encourage the use of antenatal corticosteroid therapy for fetal maturation - A randomized controlled trial. J Am Med Assoc 1999;281:46–52.
Bonanno C, Fuchs K, Wapner RJ . Single versus repeat courses of antenatal steroids to improve neonatal outcomes: risks and benefits. Obstet Gynecol Surv 2007;62:261–71.
Kemp MW, Saito M, Usuda H et al, Materno-fetal pharmacokinetics and fetal lung responses in chronically catheterised sheep receiving constant, low-dose infusions of betamethasone phosphate. Am J Obstet Gynecol 2016;215:(775): e1–12.
Keens TG, Bryan AC, Levison H, Ianuzzo CD . Developmental pattern of muscle in human ventilatory muscles fiber types. J Appl Physiol 1978;44:909–13.
West JM, Barclay CJ, Luff AR, Walker DW . Developmental changes in the activation properties and ultrastructure of fast- and slow-twitch muscles from fetal sheep. J Muscle Res Cell Motil 1999;20:249–64.
Lavin T, Song Y, Bakker AJ et al, Developmental changes in diaphragm muscle function in the preterm and postnatal lamb. Pediatr Pulmonol 2013;48:640–8.
Song Y, Pillow JJ . Ontogeny of proteolytic signaling and antioxidant capacity in fetal and neonatal diaphragm. Anat Rec 2012;295:864–71.
Song Y, Pillow JJ . Developmental regulation of molecular signalling in fetal and neonatal diaphragm protein metabolism. Exp Biol Med 2013;238:913–22.
Dekhuijzen PNR, Decramer M . Steroid-induced myopathy and its significance to respiratory disease: A known disease rediscovered. Eur Respir J 1992;5:997–1003.
Sartorelli V, Fulco M . Molecular and cellular determinants of skeletal muscle atrophy and hypertrophy. Sci STKE 2004;2004:re11.
Bonaldo P, Sandri M . Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech 2013;6:25–39.
Schakman O, Gilson H, Thissen JP . Mechanisms of glucocorticoid-induced myopathy. J Endocrinol 2008;197:1–10.
Eason JM, Dodd SL, Powers SK, Martin AD . Detrimental effects of short-term glucocorticoid use on the rat diaphragm. Phys Ther 2000;80:160–7.
Schakman O, Kalista S, Barbé C, Loumaye A, Thissen JP . Glucocorticoid-induced skeletal muscle atrophy. Int J Biochem Cell Biol 2013;45:2163–72.
Viguerie N, Picard F, Hul G et al, Multiple effects of a short-term dexamethasone treatment in human skeletal muscle and adipose tissue. Physiol Genomics 2012;44:141–51.
Song Y, Demmer DL, Pinniger GJ et al, Effect of maternal steroid on developing diaphragm integrity. PLoS One 2014;9:e93224.
Blanco CL, Moreira AG, McGill LL, Anzueto DG, Nathanielsz P, Musi N . Antenatal corticosteroids alter insulin signaling pathways in fetal baboon skeletal muscle. J Endocrinol 2014;221:253–60.
Jellyman JK, Martin-Gronert MS, Cripps RL et al, Effects of cortisol and dexamethasone on insulin signalling pathways in skeletal muscle of the ovine fetus during late gestation. PLoS ONE 2012;7:1–7.
Livak KJ, Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8.
Javen I, Williams NA, Young IR, Luff AR, Walker D . Growth and differentiation of fast and slow muscles in fetal sheep, and the effects of hypophysectomy. J Physiol 1996;494:839–49.
Liggins GC, Howie RN . A controlled trial of antepartum glucoccorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515–25.
Loehle M, Schwab M, Kadner S et al, Dose-response effects of betamethasone on maturation of the fetal sheep lung. Am J Obstet Gynecol 2010;125:2621–9.
Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham CAJ . Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem 1998;273:14484–94.
Shah OJ, Kimball SR, Jefferson LS . Among translational effectors, p70S6k is uniquely sensitive to inhibition by glucocorticoids. Biochem J 2000;347 (Pt 2): 389–97.
Savary I, Debras E, Dardevet D et al, Effect of glucocorticoid excess on skeletal muscle and heart protein synthesis in adult and old rats. Br J Nutr 1998;79:297–304.
Speirs HJL, Seckl JR, Brown RW . Ontogeny of glucocorticoid receptor and 11beta-hydroxysteroid dehydrogenase type-1 gene expression identifies potential critical periods of glucocorticoid susceptibility during development. J Endocrinol 2004;181:105–16.
Kalinyak JE, Griffin CA, Hamilton RW, Bradshaw JG, Perlman AJ, Hoffman AR . Developmental and hormonal regulation of glucocorticoid receptor messenger RNA in the rat. J Clin Invest 1989;84:1843–8.
Kuo T, Lew MJ, Mayba O, Harris CA., Speed TP, Wang JC . Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc Natl Acad Sci USA 2012;109:11160–5.
Hu Z, Wang H, In HL, Du J, Mitch WE . Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Invest 2009;119:3059–3069.
Asikainen TM, Raivio KO, Saksela M . Expression and developmental profile of antioxidant enzymes in human lung and liver. Am J Respir Cell Mol Biol 1998;19:942–949.
Geiger PC, Bailey JP, Mantilla CB, Zhan WZ, Sieck GC . Mechanisms underlying myosin heavy chain expression during development of the rat diaphragm muscle. J Appl Physiol 2006;101:1546–55.
Eizema K, van den Burg M, Kiri A et al, Differential expression of equine myosin heavy-chain mRNA and protein isoforms in a limb muscle. J Histochem Cytochem 2003;51:1207–16.
Close RI. . Dynamic mammalian properties of skeletal muscles. Physiol Rev 1972;52:129–97.
Kowalski KE, Hsieh YH, Dick TE, DiMarco AF . Diaphragm activation via high frequency spinal cord stimulation in a rodent model of spinal cord injury. Exp Neurol 2013;247:689–93.
Butler JE, Hudson AL, Gandevia SC . The neural control of human inspiratory muscles. Prog Brain Res 2014;209:295–308.
Newnham JP, Jobe AH . Should we be prescribing repeated courses of antenatal corticosteroids? Semin Fetal Neonatal Med 2009;14:157–63.
Acknowledgements
The study was supported by a National Health and Medical Research Council (NHMRC) Centre for Research Excellence (1057514), NHMRC Project Grant (1010665), NHMRC Senior Research Fellowship (1077691) and Career Development Fellowship (1045824) and the Women and Infants Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mahzabin, T., Pillow, J., Pinniger, G. et al. Influence of antenatal glucocorticoid on preterm lamb diaphragm. Pediatr Res 82, 509–517 (2017). https://doi.org/10.1038/pr.2017.99
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/pr.2017.99