Abstract
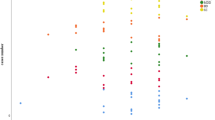
Catechol-O-methyltransferase (COMT) is an enzyme that catalyses the O-methylation, and thereby the inactivation, of catechol-containing molecules. In humans, it has been suggested that COMT modulates cognitive ability, possibly by regulating degradation of dopamine in the prefrontal cortex. Hence, it is significant that two COMT SNPs, rs4680 (c.472 G > A, p.Val158Met) and rs4818 (c.408 C > G), have been associated with cognitive ability in humans. We have shown these SNPs to be associated with levels of muscarinic M1 receptor mRNA in human cortex, which is significant as that receptor also regulates cognitive ability. We decided to determine if COMT genotype was associated with varying levels of COMT protein, as this could be a mechanism by which COMT genotype could be associated with changes in muscarinic M1 receptor mRNA levels. Hence, we measured COMT levels in prefrontal cortex obtained postmortem from 199 subjects, some of whom had a history of schizophrenia, major depressive disorders or bipolar disorders. Our data show, independent of diagnostic status, that genotype at rs4680 and rs4818, but not at rs737865 and rs165599, is associated with differing levels of soluble COMT (S-COMT), but not membrane-bound COMT (MB-COMT). These findings suggest that the association between COMT polymorphisms and cognitive functioning could be, at least in part, due to their association with varying levels of S-COMT. This is important as, unlike MB-COMT, the substrates targeted by S-COMT are likely to be intra-cellular rather than, like dopamine, located mainly in the synaptic vesicles or the extra-cellular space.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Mannisto PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51:593–628.
Barnett JH, Scoriels L, Munafò MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008;64:137–44.
Goldman-Rakic PS. The “psychic” neuron of the cerebral cortex. Ann N Y Acad Sci. 1999;868:13–26.
Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–22.
Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, et al. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry. 2002;52:701–7.
Shifman S, Bronstein M, Sternfeld M, Pisanté-Shalom A, Lev-Lehman E, Weizman A, et al. A highly significant association between a COMT haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–302.
Ulmanen I, Peränen J, Tenhunen J, Tilgman C, Karhunen T, Panula P, et al. Expression and intracellular localization of catechol O-methyltransferase in transfected mammalian cells. Eur J Biochem. 1997;243:452–9.
Lundstrom K, Salminen M, Jalanko A, Savolainen R, Ulmanen I. Cloning and characterization of human placental catechol-O-methyltransferase cDNA. DNA Cell Biol. 1991;10:181–9.
Chen J, Song J, Yuan P, Tian Q, Ji Y, Ren-Patterson R, et al. Orientation and cellular distribution of membrane-bound catechol-O-methyltransferase in cortical neurons: implications for drug development. J Biol Chem. 2011;M111:262790. jbc
Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melén K, Julkunen U, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–10.
Tenhunen J, Salminen M, Lundström K, Kiviluoto T, Savolainen R, Ulmanen I. Genomic organization of the human catechol-O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem. 1994;347:1049–59.
Lundström K, Tenhunen J, Tilgmann C, Karhunen T, Panula P, Ulmanen I. Cloning, expression and structure of catechol-O-methyltransferase. Biochim Biophys Acta. 1995;1251:1–10.
Tenhunen J, Salminen M, Jalanko A, Ukkonen A, Ulmanen I. Structure of the rat catechol-O-methyltransferase gene: separate promoters are used to produce mRNAs for soluble and membrane-bound forms of the enzyme. DNA Cell Biol. 1993;12:253–63.
Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, et al. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology. 2003;28:1521–30.
Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–21.
Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–50.
Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–3.
Roussos P, Giakoumaki SG, Pavlakis S, Bitsios P. Planning, decision-making and the COMT rs4818 polymorphism in healthy males. Neuropsychologia. 2008;46:757–63.
Matsuzaka CT, Christofolini D, Ota VK, Gadelha A, Berberian AA, Noto C, et al. Catechol-O-methyltransferase (COMT) polymorphisms modulate working memory in individuals with schizophrenia and healthy controls. Rev Bras Psiquiatr. 2017;39:302–8.
Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, et al. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am J Hum Genet. 2003;73:152–61.
Dean B, Scarr E. COMT genotype is associated with differential expression of muscarinic M1 receptors in human cortex. Am J Med Genet B Neuropsychiatr Genet. 2016;171:784–9.
Scarr E, Dean B. Muscarinic receptors: do they have a role in the pathology and treatment of schizophrenia? J Neurochem. 2008;107:1188–95.
Ferrer I, Santpere G, Arzberger T, Bell J, Blanco R, Boluda S, et al. Brain protein preservation largely depends on the postmortem storage temperature: implications for study of proteins in human neurologic diseases and management of brain banks: a BrainNet Europe Study. J Neuropathol Exp Neurol. 2007;66:35–46.
Dean B, Pavey G, Chai SY, Mendelsohn FAO. The localisation and quantification of molecular changes in the human brain using in situ radioligand binding and autoradiography. In: Dean B, Kleinman JE, Hyde TM, editors. Using CNS tissue in psychiatric research: a practical guide. Amsterdam: Harwood Academic; 1999. p. 67–83.
Stan AD, Ghose S, Gao XM, Roberts RC, Lewis-Amezcua K, Hatanpaa KJ, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11.
Kingsbury AE, Foster OJ, Nisbet AP, Cairns N, Bray L, Eve DJ, et al. Tissue pH as an indicator of mRNA preservation in human post-mortem brain. Brain Res Mol Brain Res. 1995;28:311–8.
Hill C, Keks N, Roberts S, Opeskin K, Dean B, MacKinnon A, et al. Problem of diagnosis in postmortem brain studies of schizophrenia. Am J Psychiatry. 1996;153:533–7.
Roberts SB, Hill CA, Dean B, Keks NA, Opeskin K, Copolov DL. Confirmation of the diagnosis of schizophrenia after death using DSM-IV: a Victorian experience. Aust N Z J Psychiatry. 1998;32:73–76.
Rodriguez S, Gaunt TR, Day IN. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol. 2009;169:505–14.
Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5.
Quade D. Rank analysis of covariance. J Am Stat Assoc. 1967;62:1187–1200.
Cook RD, Weisberg S. Applied regression including computing and graphics. Wiley-Interscience: New York, NY, USA, 1999.
Ratner B. The correlation coefficient: its values range between + 1/− 1, or do they? J Target, Meas Anal Mark. 2009;17:139–42.
Tunbridge E, Weickert C, Kleinman J, Herman M, Chen J, Kolachana B, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex. 2006;17:1206–12.
Strous RD, Lapidus R, Viglin D, Kotler M, Lachman HM. Analysis of an association between the COMT polymorphism and clinical symptomatology in schizophrenia. Neurosci Lett. 2006;393:170–3.
Tsai SJ, Hong CJ, Hou SJ, Yen FC. Lack of association of catechol-O-methyltransferase gene Val108/158Met polymorphism with schizophrenia: a family-based association study in a Chinese population. Mol Psychiatry. 2006;11:2–3.
Fan JB, Zhang CS, Gu NF, Li XW, Sun WW, Wang HY, et al. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol Psychiatry. 2005;57:139–44.
Lautala P, Ulmanen I, Taskinen J. Molecular mechanisms controlling the rate and specificity of catechol O-methylation by human soluble catechol O-methyltransferase. Mol Pharmacol. 2001;59:393–402.
Bertocci B, Miggiano V, Da Prada M, Dembic Z, Lahm HW, Malherbe P. Human catechol-O-methyltransferase: cloning and expression of the membrane-associated form. Proc Natl Acad Sci USA. 1991;88:1416–20.
Reenilä I, Männistö PT. Catecholamine metabolism in the brain by membrane-bound and soluble catechol-O-methyltransferase (COMT) estimated by enzyme kinetic values. Med Hypotheses. 2001;57:628–32.
Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–6.
Babovic D, O’tuathaigh C, O’connor A, O’sullivan G, Tighe O, Croke D, et al. Phenotypic characterization of cognition and social behavior in mice with heterozygous versus homozygous deletion of catechol-O-methyltransferase. Neuroscience. 2008;155:1021–9.
Schütze N, Vollmer G, Knuppen R. Catecholestrogens are agonists of estrogen receptor dependent gene expression in MCF-7 cells. J Steroid Biochem Mol Biol. 1994;48:453–61.
Cardoso CC, Ricardo VP, Frussa-Filho R, Porto CS, Abdalla FMF. Effects of 17β-estradiol on expression of muscarinic acetylcholine receptor subtypes and estrogen receptor α in rat hippocampus. Eur J Pharmacol. 2010;634:192–200.
Arevalo M-A, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16:17.
Melancon BJ, Tarr JC, Panarese JD, Wood MR, Lindsley CW. Allosteric modulation of the M1 muscarinic acetylcholine receptor: improving cognition and a potential treatment for schizophrenia and Alzheimer’s disease. Drug Discov Today. 2013;18:1185–99.
Papaleo F, Sannino S, Piras F, Spalletta G. Sex-dichotomous effects of functional COMT genetic variations on cognitive functions disappear after menopause in both health and schizophrenia. Eur Neuropsychopharmacol. 2015;25:2349–63.
Acknowledgements
The authors would like to thank Mr. Geoff Pavey for careful dissection of the tissue that was used in this study. This project was supported by the National Health and Medical Research Council (NHMRC; Australia; project grant 566967), the Cooperative Research Centre (CRC) for Mental Health and the Victorian Government’s Operational Infrastructure Support Programme. BD is a recipient of NHMRC Fellowship APP1002240, ES was supported by the Australian Research Council (Future Fellowship FT100100689) and GMP is an awardee of an Australian Government Research Training Program Scholarship. The Victorian Brain Bank Network is supported by The Florey Institute of Neuroscience and Mental Health and the Alfred and the Victorian Forensic Institute of Medicine, and funded in part by Parkinson’s Victoria and MND Victoria and Mental Health.
Funding
This project was supported by the National Health and Medical Research Council (NHMRC; Australia; project grant 566967), the Cooperative Research Centre (CRC) for Mental Health and the Victorian Government’s Operational Infrastructure Support Programme. GMP is an awardee of an Australian Government Research Training Program Scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Parkin, G.M., Udawela, M., Gibbons, A. et al. Catechol-O-methyltransferase (COMT) genotypes are associated with varying soluble, but not membrane-bound COMT protein in the human prefrontal cortex. J Hum Genet 63, 1251–1258 (2018). https://doi.org/10.1038/s10038-018-0511-2
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-018-0511-2
This article is cited by
-
Genetic and epigenetic regulation of Catechol-O-methyltransferase in relation to inflammation in chronic fatigue syndrome and Fibromyalgia
Journal of Translational Medicine (2022)
-
Changes in cortical gene expression in the muscarinic M1 receptor knockout mouse: potential relevance to schizophrenia, Alzheimer’s disease and cognition
npj Schizophrenia (2021)
-
Associations between catechol-O-methyltransferase (COMT) genotypes at rs4818 and rs4680 and gene expression in human dorsolateral prefrontal cortex
Experimental Brain Research (2020)