Abstract
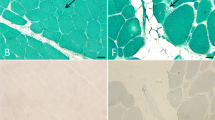
Reducing body myopathy is a rare X-linked myopathy characterized by the presence of reducing bodies. The causative gene has been identified as FHL1. We presented with the clinical, muscle magnetic resonance imaging and genetic features of 6 unrelated Chinese patients with reducing body myopathy. We divided the patients into 2 groups according to their age at onset. In addition to limb muscle weakness, pronounced axial muscle involvement was a striking feature common to both groups. Muscle magnetic resonance imaging revealed fatty infiltration predominantly in the postero-medial muscles of the thigh and the soleus muscle of the calf, sparing the gluteus and sartorius muscles. Muscle pathology demonstrated the muscle fibres with reducing bodies distributed in small groups. Genetic analysis revealed FHL1 hemizygote variants in the 6 patients, including 4 novel and 2 reported variants. These variants were located in the LIM2 domain of FHL1 in 4 patients, but 2 located in the LIM4 domain. To the best of our knowledge, this is the first report of reducing body myopathy in the Chinese population. Our findings expand the genetic spectrum of reducing body myopathy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
Supplementary information is available at Jounal of human genetics’s website.
References
Schessl J, Taratuto AL, Sewry C, Battini R, Chin SS, Maiti B, et al. Clinical, histological and genetic characterization of reducing body myopathy caused by mutations in FHL1. Brain. 2009;132:452–64.
Shalaby S, Hayashi YK, Nonaka I, Noguchi S, Nishino I. Novel FHL1 mutations in fatal and benign reducing body myopathy. Neurology. 2009;72:375–6.
Schessl J, Columbus A, Hu Y, Zou Y, Voit T, Goebel HH, et al. Familial reducing body myopathy with cytoplasmic bodies and rigid spine revisited: identification of a second LIM domain mutation in FHL1. Neuropediatrics. 2010;41:43–6.
Malfatti E, Olivé M, Taratuto AL, Richard P, Brochier G, Bitoun M, et al. Skeletal muscle biopsy analysis in reducing body myopathy and other FHL1-related disorders. J Neuropathol Exp Neurol. 2013;72:833–45.
Schreckenbach T, Henn W, Kress W, Roos A, Maschke M, Feiden W, et al. Novel FHL1 mutation in a family with reducing body myopathy. Muscle Nerve. 2013;47:127–34.
Fujii T, Hayashi S, Kawamura N, Higuchi MA, Tsugawa J, Ohyagi Y, et al. A case of adult-onset reducing body myopathy presenting a novel clinical feature, asymmetrical involvement of the sternocleidomastoid and trapezius muscles. J Neurol Sci. 2014;343:206–10.
Sabatelli P, Castagnaro S, Tagliavini F, Chrisam M, Sardone F, Demay L, et al. Aggresome-autophagy involvement in a sarcopenic patient with rigid spine syndrome and a p.C150R mutation in FHL1 gene. Front Aging Neurosci. 2014;6:215.
Windpassinger C, Schoser B, Straub V, Noor A, Lohberger B, Farra N, et al. An X-linked myopathy with postural muscle atrophy and generalized hypertrophy, termed XMPMA, is caused by mutations in FHL1. Am J Hum Genet. 2008;82:88–9.
Quinzii CM, Vu TH, Min KC, Tanji K, Barral S, Grewal RP, et al. X-linked dominant scapuloperoneal myopathy is due to a mutation in the gene encoding four-and-a-half-LIM protein 1. Am J Hum Genet. 2008;82:208–13.
D’Arcy C, Kanellakis V, Forbes R, Wilding B, McGrath M, Howell K, et al. X-linked recessive distal myopathy with hypertrophic cardiomyopathy caused by a novel mutation in the FHL1 gene. Child Neurol. 2015;30:1211–7.
Xue Y, Schoser B, Rao AR, Quadrelli R, Vaglio A, Rupp V, et al. Exome sequencing identified a splice site mutation in FHL1 that causes uruguay syndrome, an X-linked disorder with skeletal muscle hypertrophy and premature cardiac death. Circ Cardiovasc Genet. 2016;9:130–5.
Pen AE, Nyegaard M, Fang M, Jiang H, Christensen R, Mølgaard H, et al. A novel single nucleotide splice site mutation in FHL1 confirms an Emery-Dreifuss plus phenotype with pulmonary artery hypoplasia and facial dysmorphology. Eur J Med Genet. 2015;58:222–9.
Hartmannova H, Kubanek M, Sramko M, Piherova L, Noskova L, Hodanova K, et al. Isolated X-linked hypertrophic cardiomyopathy caused by a novel mutation of the four-and-a-half LIM domain 1 gene. Circ Cardiovasc Genet. 2013;6:543–51.
Gossios TD, Lopes LR, Elliott PM. Left ventricular hypertrophy caused by a novel nonsense mutation in FHL1. Eur J Med Genet. 2013;56:251–5.
Gueneau L, Bertrand AT, Jais JP, Salih MA, Stojkovic T, Wehnert M, et al. Mutations of the FHL1 gene cause Emery-Dreifuss muscular dystrophy. Am J Hum Genet. 2009;85:338–53.
Brooke MH, Neville HE. Reducing body myopathy. Neurology. 1972;22:829–40.
Chen DH, Raskind WH, Parson WW, Sonnen JA, Vu T, Zheng Y, et al. A novel mutation in FHL1 in a family with X-linked scapuloperoneal myopathy: phenotypic spectrum and structural study of FHL1 mutations. J Neurol Sci. 2010;296:22–9.
Knoblauch H, Geier C, Adams S, Budde B, Rudolph A, Zacharias U, et al. Contractures and hypertrophic eardiomyopathy in a novel FHLl mutation. Ann Neurol. 2010;67:136–40.
Shalaby S, Hayashi YK, Goto K, Ogawa M, Nonaka I, Noguchi S, et al. Rigid spine syndrome caused by a novel mutation in four-and-a-half LIM domain 1 gene (FHL1). Neuromuscul Disord. 2008;18:959–61.
Selcen D, Bromberg MB, Chin SS, Engel AG. Reducing bodies and myofibrillar myopathy features in FHL1 muscular dystrophy. Neurology. 2011;77:1951–9.
Feldkirchner S, Walter MC, Muller S, Kubny C, Krause S, Kress W, et al. Proteomic characterization of aggregate components in an intrafamilial variable FHL1-associated myopathy. Neuromuscul Disord. 2013;23:418–26.
Astrea G, Schessl J, Clement E, Tosetti M, Mercuri E, Rutherford M, et al. Muscle MRI in FHL1-linked reducing body myopathy. Neuromuscul Disord. 2009;19:689–91.
Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F, et al. Muscle MRI in inherited neuromuscular disorders:past, present and future. J Magn Reson Imaging. 2007;25:433–40.
Nali M, Areehavala-Gomcza V, Cirak S, Glover A, Guglieri M, Feng L, et al. Muscle histology VS MRI in Duchenne muscular dystrophy. Neurology. 2011;76:346–53.
Stramare R, Beltrame V, Dal Borgo R, Gallimberti L, Frigo AC, Pegoraro E, et al. MRI in the assessment of muscular pathology: a comparison between limb-girdle muscular dystrophies, hyaline body myopathies and myotonic dystrophies. Radio Med. 2010;115:585–99.
Waddell LB, Tran J, Zheng XF, Bönnemann CG, Hu Y, Evesson FJ, et al. A study of FHL1, BAG3, MATR3, PTRF and TCAP in Australian muscular dystrophy patients. Neuromuscul Disord. 2011;21:776–8.
Selcen D. Myofibrillar myopathies. Curr Opin Neurol. 2008;21:585–9.
Cowling BS, Cottle DL, Wilding BR, D’Arcy CE, Mitchell CA, McGrath MJ. Four and a half LIM protein 1 gene mutations cause four distinct human myopathies: a comprehensive review of the clinical, histological and pathological features. Neuromusclar Disord. 2011;21:237–51.
Bertrand AT, Bonnemann CG, Bonne G. FHL1 myopathy consortium. 199th ENMC international workshop: FHL1 related myopathies, June 7-9, 2013, Naarden, The Netherlands. Neuromuscul Disord: NMD. 2014;24:453–62.
Schoser B, Goebel HH, Janisch I, Quasthoff S, Rother J, Bergmann M, et al. Consequences of mutations within the C terminus of the FHL1 gene. Neurology. 2009;73:543–51.
Schessl J, Zou Y, McGrath MJ, Cowling BS, Maiti B, Chin SS, et al. Proteomic identification of FHL1 as the protein mutated in human reducing body myopathy. J Clin Invest. 2008;118:904–12.
Komagamine T, Kawai M, Kokubun N, Miyatake S, Ogata K, Hayashi YK, et al. Selective muscle involvement in a family affected by a second LIM domain mutation offhl1: an imaging study using computed tomography. J Neurol Sci. 2012;318:163–7.
Acknowledgements
We extend our sincere appreciation to the patients and their parents for their participation and enthusiastic support. We thank Ichizo Nishino (National Institute of Neuroscience, NCNP) for reviewing and commenting on the manuscript.
Funding
This work was financially supported by grants from the Ministry of Science and Technology of China (No. 2011ZX09307-001-07), the National Natural Science Foundation of China (No. 81541118) and the Beijing Municipal Science and Technology Commission (No. Z151100003915126).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hu, Z., Zhu, Y., Liu, X. et al. FHL1-related clinical, muscle MRI and genetic features in six Chinese patients with reducing body myopathy. J Hum Genet 64, 919–926 (2019). https://doi.org/10.1038/s10038-019-0627-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s10038-019-0627-z
This article is cited by
-
French National Protocol for diagnosis and care of facioscapulohumeral muscular dystrophy (FSHD)
Journal of Neurology (2024)
-
FHL1 promotes chikungunya and o’nyong-nyong virus infection and pathogenesis with implications for alphavirus vaccine design
Nature Communications (2023)
-
Identification of novel FHL1 mutations associated with X-linked scapuloperoneal myopathy in unrelated Chinese patients
Journal of Human Genetics (2023)
-
Clinical, pathological, and molecular genetic analysis of 7 Chinese patients with hereditary myopathy with early respiratory failure
Neurological Sciences (2022)
-
A Dominant C150Y Mutation in FHL1 Induces Structural Alterations in LIM2 Domain Causing Protein Aggregation In Human and Drosophila Indirect Flight Muscles
Journal of Molecular Neuroscience (2021)