Abstract
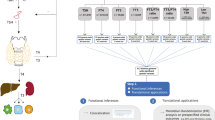
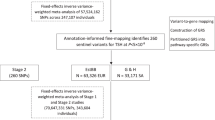
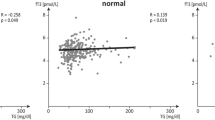
Thyroid hormones are central to regulating metabolism, growth, and development, yet their complex interactions with socioeconomic, metabolic, and genetic factors remain understudied in diverse populations. We compared thyroid profiles - free triiodothyronine (FT3), free thyroxine (FT4), and thyroid-stimulating hormone (TSH) in Indian adolescents with anthropometric traits, metabolic markers, and socioeconomic status (SES). We observed that adolescents from higher SES backgrounds exhibited greater metabolic dysregulation, altered thyroid profiles, and abnormalities in lipid and adipokine levels. Subclinical (16.1%) and clinical hypothyroidism (1.1%) were found to be prevalent in this population but were not associated with obesity. Instead, they showed links with dyslipidemia and altered adipokine profiles. To investigate the genetic basis of thyroid traits, we conducted an exome-wide association study (ExWAS, N = 4324), and a two-staged genome-wide association study (GWAS, N = 4854). The ExWAS revealed two novel loci for TSH (GYS2 and CEP162) and fifteen novel loci for FT4, including ZNF467, P3H3, CRLF3, SPATA2L, MEFV, THNSL2, COL27A1, COL28A1, IGSF3, ZNF732, MOG, GABBR1, HPF1, LOC440563, and SPEG. The GWAS identified novel associations at near-genome-wide significance for TSH (ACTL7B) and FT4 (LINC00648, YTHDC1, and C2CD4B). We also replicated established associations in FOXE1 and IGFBP5. Our findings suggest that SES, metabolic health, and genetics jointly influence thyroid function in Indian adolescents. The identification of population-specific loci emphasizes the importance of ancestry-informed genetic studies and supports the development of precision interventions to enhance pediatric thyroid health.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Taylor PN, Razvi S, Pearce SH, Dayan CM. A Review of the Clinical Consequences of Variation in Thyroid Function Within the Reference Range. J Clin Endocrinol Metab. 2013;98:3562–71.
Panicker V. Genetics of thyroid function and disease. Clin Biochem Rev. 2011;32:165–75.
Sterenborg RBTM, Steinbrenner I, Li Y, Bujnis MN, Naito T, Marouli E, et al. Multi-trait analysis characterizes the genetics of thyroid function and identifies causal associations with clinical implications. Nat Commun. 2024;15:888.
Kwak SH, Park YJ, Go MJ, Lee KE, Kim SJ, Choi HS, et al. A genome-wide association study on thyroid function and anti-thyroid peroxidase antibodies in Koreans. Hum Mol Genet. 2014;23:4433–42.
Unnikrishnan A, Kalra S, Sahay R, Bantwal G, John M, Tewari N. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J Endocrinol Metab. 2013;17:647.
Ministry of Health and Family Welfare. Status of Goitre or Thyroid Disorders in India. 2022.
Marwaha RK, Tandon N, Desai A, Kanwar R, Grewal K, Aggarwal R, et al. Reference range of thyroid hormones in normal Indian school-age children. Clin Endocrinol. 2008;68:369–74.
Marwaha RK, Tandon N, Gupta N, Karak AK, Verma K, Kochupillai N. Residual goitre in the postiodization phase: iodine status, thiocyanate exposure and autoimmunity. Clin Endocrinol. 2003;59:672–81.
Ministry of Health and Family Welfare G. National Family health Survey (NFHS-5), 2019-21, India Report. 2024. Available from: http://www.rchiips.org/nfhs.
Marwaha RK, Tandon N, Agarwal N, Puri S, Agarwal R, Singh S, et al. Impact of two regimens of vitamin D supplementation on calcium — vitamin D — PTH axis of schoolgirls of Delhi. Indian Pediatr. 2010;47:761–9.
Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes. 2012;7:284–94.
Vigone MC, Capalbo D, Weber G, Salerno M. Mild Hypothyroidism in Childhood: Who, When, and How Should Be Treated?. J Endocr Soc. 2018;2:1024–39.
Tabassum R, Mahendran Y, Dwivedi OP, Chauhan G, Ghosh S, Marwaha RK, et al. Common variants of IL6, LEPR, and PBEF1 are associated with obesity in Indian children. Diabetes. 2012;61:626–31.
Nair JM, Chauhan G, Prasad G, Bandesh K, Giri AK, Chakraborty S, et al. Mapping the landscape of childhood obesity: genomic insights and socioeconomic status in Indian school-going children. Obesity. 2025;33:754–65.
Patterson N, Price AL, Reich D. Population Structure and Eigenanalysis. PLoS Genet. 2006;2:e190.
Taliun D, Harris DN, Kessler MD, Carlson J, Szpiech ZA, Torres R, et al. Sequencing of 53,831 diverse genomes from the NHLBI TOPMed Program. Nature. 2021;590:290–9.
Fuchsberger C, Abecasis GR, Hinds DA. minimac2: faster genotype imputation. Bioinformatics. 2015;31:782–4.
Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–1.
Lonsdale J, Thomas J, Salvatore M, Phillips R, Lo E, Shad S, et al. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013;45:580–5.
Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8:1826.
Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6:80–92.
Cingolani P, Patel VM, Coon M, Nguyen T, Land SJ, Ruden DM, et al. Using Drosophila melanogaster as a Model for Genotoxic Chemical Mutational Studies with a New Program, SnpSift. Front Genet. 2012;3:35.
Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, et al. A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function. PLoS Genet. 2013;9:e1003266.
Baksi S, Pradhan A. Thyroid hormone: sex-dependent role in nervous system regulation and disease. Biol Sex Differ. 2021;12:25.
Jia X, Zhai T, Wang B, Zhang J, Zhang F. The MAGI2 gene polymorphism rs2160322 is associated with Graves’ disease but not with Hashimoto’s thyroiditis. J Endocrinol Invest. 2019;42:843–50.
Owens PW, McVeigh TP, Miller N, Guerin C, Sebag F, Quill D, et al. FOXE1 polymorphism rs965513 predisposes to thyroid cancer in a European cohort. Endocrine Oncol. 2021;1:1–8.
Denny JC, Crawford DC, Ritchie MD, Bielinski SJ, Basford MA, Bradford Y, et al. Variants Near FOXE1 Are Associated with Hypothyroidism and Other Thyroid Conditions: Using Electronic Medical Records for Genome- and Phenome-wide Studies. Am J Hum Genet. 2011;89:529–42.
Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–7.
Zhou Y, Huang X, Wang L, Luo Y. The Expression Characteristics and Function of the RECQ Family in Pan-Cancer. Biomedicines. 2023;11:2318.
Yao X, Hou S, Zhang D, Xia H, Wang YC, Jiang J, et al. Regulation of fatty acid composition and lipid storage by thyroid hormone in mouse liver. Cell Biosci. 2014;4:38.
Rechtman A, Zveik O, Haham N, Freidman-Korn T, Vaknin-Dembinsky A. Thyroid hormone dysfunction in MOGAD and other demyelinating diseases. J Neurol Sci. 2024;457:122866.
Qin Y, Sun W, Wang Z, Dong W, He L, Zhang T, et al. ATF2-Induced lncRNA GAS8-AS1 Promotes Autophagy of Thyroid Cancer Cells by Targeting the miR-187-3p/ATG5 and miR-1343-3p/ATG7 Axes. Mol Ther Nucleic Acids. 2020;22:584–600.
Di Maro G, Orlandella FM, Bencivenga TC, Salerno P, Ugolini C, Basolo F, et al. Identification of Targets of Twist1 Transcription Factor in Thyroid Cancer Cells. J Clin Endocrinol Metab. 2014;99:E1617–26.
Gulcan E, Gulcan A, Koplay M, Alcelik A, Korkmaz U. Co-existence of Hashimoto’s thyroiditis with familial Mediterranean fever: is there a pathophysiological association between the two diseases?. Clin Exp Immunol. 2009;156:373–6.
Zhang Y, Jin T, Shen H, Yan J, Guan M, Jin X. Identification of Long Non-Coding RNA Expression Profiles and Co-Expression Genes in Thyroid Carcinoma Based on The Cancer Genome Atlas (TCGA) Database. Medical Sci Monit. 2019;25:9752–69.
Zhou Q, Tian M, Cao Y, Tang M, Xiang X, Guo L, et al. YTHDC1 aggravates high glucose-induced retinal vascular endothelial cell injury via m6A modification of CDK6. Biol Direct. 2024;19:54.
Di Pietro P, Abate AC, Prete V, Damato A, Venturini E, Rusciano MR, et al. C2CD4B Evokes Oxidative Stress and Vascular Dysfunction via a PI3K/Akt/PKCα–Signaling Pathway. Antioxidants. 2024;13:101.
Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018;14:259–69.
Laxmaiah A, Arlappa N, Balakrishna N, Mallikarjuna Rao K, Galreddy C, Kumar S, et al. Prevalence and Determinants of Micronutrient Deficiencies among Rural Children of Eight States in India. Ann Nutr Metab. 2013;62:231–41.
Pandav CS, Bajaj S, Yadav K, Joshi SR, Seshadri KG, Kalra P, et al. Indian Thyroid society expert consensus on salt Iodisation. Thyroid Res Pract. 2024;20:59–63.
Johner SA, Thamm M, Stehle P, Nöthlings U, Kriener E, Völzke H, et al. Interrelations Between Thyrotropin Levels and Iodine Status in Thyroid-Healthy Children. Thyroid. 2014;24:1071–9.
Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3:286–95.
Fernández LP, López-Márquez A, Martínez ÁM, Gómez-López G, Santisteban P. New Insights into FoxE1 Functions: Identification of Direct FoxE1 Targets in Thyroid Cells. PLoS One. 2013;8:e62849.
Xuesong L, Chuanwei L, Zheng H, Zhilin Z, Yiling Q, Yu Z, et al. IGFBP5 protein molecule and thyroid hormone in the diagnosis and effect of gestational diabetes. Int J Biol Macromol. 2025;309:142737.
Boucai L, Hollowell JG, Surks MI. An Approach for Development of Age-, Gender-, and Ethnicity-Specific Thyrotropin Reference Limits. Thyroid. 2011;21:5–11.
Acknowledgements
The authors thank all the students and their families for their kind co-operation and participation in the study. Special acknowledgment to Praveen Gupta, MD, Premas Life Sciences Pvt. Ltd., for always providing help whenever required. J.M.N thanks the Council of Scientific and Industrial Research, Government of India for the Senior Research Fellowship. SC thanks the Department of Science and Technology, Govt. of India for INSPIRE faculty fellowship.
Funding
Major funding for this study was sponsored by the Department of Biotechnology, Government of India under two projects: ‘Genetics and systems biology of childhood obesity in India and Denmark’ (BIOCHILD) [GAP 0089] and ‘Childhood Obesity: inflammatory markers, gene variation and epigenetics’ (GLUE) [N 1292]. Partial funding for this study was also granted by the Department of Science & Technology, Government of India (PURSE II CDST/SR/PURSE PHASE II/11).
Author information
Authors and Affiliations
Contributions
JMN: Literature search, visualization, data analysis, data interpretation, writing; KB: Data collection, intellectual inputs; AKG: Data collection, data curation, data analysis, intellectual inputs; RKM: Sample collection; AB: Study design, conceptualization, methodology, statistical analysis; NT: Sample collection, and phenotyping; SC: Data collection, data curation, data analysis, writing, interpretation, intellectual inputs; DB: Study design, conceptualization, methodology, funding acquisition, investigation, and supervised the entire study. DB is the guarantor of this work and, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nair, J.M., Bandesh, K., K. Giri, A. et al. Exploring socio-economic, biochemical, and genetic factors influencing thyroid status in Indian school-going adolescents. J Hum Genet (2025). https://doi.org/10.1038/s10038-025-01432-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s10038-025-01432-z