Abstract
The coupled effects of freeze-thaw cycles and salt are key drivers of deterioration in earthen heritage site. This study investigates the influence of cooling rate on deterioration by conducting simulated freeze-thaw cycles on salt-contaminated earth specimens. Macro- and micro-deterioration characteristics, along with salt crystallization behavior, were analyzed to elucidate the mechanism by which cooling rates induce pore damage through water-salt phase transformation. Results indicate that NaCl causes more severe damage than Na₂SO₄ during freeze-thaw cycles, with the extent of deterioration increasing at slower cooling rates. Salt and earth particles coagulate into aggregates, increasing surface strength, an effect is more pronounced at slower cooling rates. Additionally, cooling rates affect the distribution and crystal growth of salts, with slower rates forming larger and more complete crystals. These findings enhance understanding of the deterioration process and provide valuable insights for conservation strategies to mitigate freeze-thaw and salt-induced damage in earthen sites.
Similar content being viewed by others
Introduction
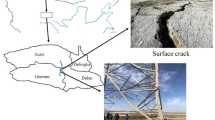
Earthen sites, remnants of historical human activities, are indispensable component of cultural heritage, preserving invaluable historical information1,2. However, the conservation of these sites presents significant challenges, particularly for those located in extreme environments. In China, more than 70% of earthen sites are situated in region with frozen soil ground as shown in Fig. 1. Among these, saline soil sites are especially susceptible to damage due to the repeated dissolution-crystallization cycles of salts induced by freeze-thaw cycles. This results in substantial reductions in mechanical strength and significant changes in physical and chemical properties of soils3,4. These processes accelerate the development of various forms of deterioration, including peeling, sapping, etc. (Fig. 2) Consequently, investigating the deterioration mechanisms of earthen sites under the combined effects of freeze-thaw cycles and salt activity is crucial for mitigating weathering and implementing effective preventive conservation strategies.
(The dataset is provided by National Cryosphere Desert Data Center39).
Research on the freeze-thaw degradation of saline soils has evolved through several stages over the years. From the late 20th century to the early 21st century, studies primarily focused on the impact of freeze-thaw cycles on the basic physical and mechanical properties of saline soils5,6. From early 21st century up to the 2010s, researches advanced to investigate the migration of water and salts during freeze-thaw processes, employing simulation experiments and numerical modeling7,8,9,10. These studies identified temperature gradients and moisture migration as key factors influencing salt redistribution11. Since around 2010, attention has shifted toward exploring soil degradation mechanisms under the combined influence of multiple factors, with an emphasis on uncovering the underlying causes at the microstructural level12. It is now widely recognized that pore structure damage caused by crystallization pressure, resulting from salt precipitation due to supersaturation during freezing, is the fundamental mechanism driving soil deformation and degradation13,14. Among the factors influencing salt crystallization during freezing, the cooling rate is particularly crucial. It significantly affects the nucleation type, crystal morphology, crystallization rate, and expansion rate, ultimately resulting in variations in the freezing-induced deformation characteristics of the soil15,16.
Regarding earthen sites, research on the coupled effects of salt and freeze-thaw has predominantly focused on macroscopic deterioration patterns and microstructural pore changes. At the macro level, it has been established that freeze-thaw cycles and salinization are the primary drivers of microstructural damage in rammed earth sites, with surface peeling being the most common form of deterioration caused by snowmelt17,18. To better guide the preservation of more sites, a susceptibility map for freeze-thaw deterioration of earthen sites in China has been developed19. At the micro-mechanism level, studies have investigated the changes in the microstructure of saline soils under freeze-thaw cycles3, examining how different initial conditions and temperature ranges influence the progression of deterioration20,21,22,23. However, there have been few comprehensive study attempts to elucidate the mechanisms from the perspective of salt crystallization, and research specifically addressing the critical factor of cooling rate remains limited. Moreover, due to the wide geographic distribution and environmental variability of earthen sites, freezing cooling rates vary significantly across regions. These variations affect the phase transitions of water and salts within the soil, leading to differences in deterioration characteristics. Therefore, investigating the impact of cooling rate on the freeze-thaw of saline soils containing mixed salt is essential for understanding specific patterns and identifying threshold conditions for deterioration. Such research would provide deeper and more comprehensive insights into the mechanisms.
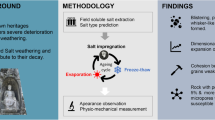
In this study, Na2SO4-NaCl was selected to prepare remolded specimens. The freeze-thaw cycle simulation test of salt-containing site soil was carried out with different cooling rates. The macroscopic strength, microstructure and salt crystallization characteristics were tested to explore the mechanism of pore damage caused by water-salt phase transition, which eventually induce obvious deterioration. Furthermore, this study aims to contribute valuable insights for the development of anti-weathering conservation strategies and preventive measures for earthen heritage that are subjected to the combined effects of salt and freeze-thaw cycles.
Methods
Sampling location and soil properties
The test soil was taken from the vicinity of the Ming Great Wall site in northern Shaanxi, and its basic physical properties were similar to those of the site soil. The test results are shown in Table 1. Specifically, the particle size distribution of the soil specimens predominantly ranged from 0.005 mm to 0.075 mm, with silt particles being more abundant than clay particles, classifying it as silty clay. This composition is representative of the soil characteristics commonly found at earthen sites in northern China, such as the Great Wall site in Jiuquan, Jiaohe Relics site, etc24. With regards to salinity, the test soil contained approximately 0.09% salt (refer to Table 2), a concentration that does not classify it as saline soil. Consequently, no desalination treatment was deemed necessary for the soil. Analysis of the ionic composition revealed a cation hierarchy of Na+ > Mg2+ > K+ > Ca2+, while the anion content was dominated by SO42- > Cl- > NO3-.
Remolded soil sample preparation
Salt solution and pure water in different proportions were used as pore solution (Table 3) to prepare salt-containing remolded soil specimens. The selection of mixed salt types is decided by extensive field investigations. Since freeze-thaw cycles predominantly affect earthen sites in northern China, where sodium sulfate and sodium chloride have been identified as the most destructive salts25. Prominent examples of such damage include the Site of Yumen Pass and the Han Great Wall Ruins26. To investigate the destructive effects of these two salts more precisely, experiments were conducted using pure Na2SO4, pure NaCl, and mixed salts with varying proportions of them. The selection of mixing ratios was based on the literature27,28, which indicates that in the Na₂SO₄–NaCl–H₂O system, significant differences in crystallization pathways occur when the molar ratio of NaCl is 0.33, 0.5, and 0.8. Sample preparation adhered to the Standard for Geotechnical Testing Methods29. According to the compaction test results, the dry density of the soil is determined as 1.7 g/cm3, with the moisture content of 11%. The prepared samples were cylindrical, with both the diameter and height measuring 5 cm.
Freeze-thaw cycles treatment
The temperature gradient of the freeze-thaw cycle is 25 °C– −5 °C, which refers to the real environment of the Ming Great Wall. Three different cooling rates of 0.1 °C/min, 0.5 °C/min and 1 °C/min were set up, and a freeze-thaw cycle was performed every 24 h for 15 cycles. Each cycle consists of two stages: (1) Freezing stage: the specimen is placed in the environmental chamber, and the temperature is cooled at a certain rate from the initial 25 °C to −5 °C, and then remaining under −5 °C for 12 h. (2) Melting stage: The specimens were taken out from the low temperature environment and placed at room temperature of 25 °C for 12 h.
Macroscopic deterioration test
(1) Morphology. The morphology change characteristics were photographed and recorded during the freeze-thaw process. (2) Drilling resistance test. Subsequent to the completion of the freeze-thaw cycle, a drilling resistance test was conducted with a standardized drilling depth of 15 mm. The test was executed at a drilling speed of 40 mm/min and a rotation speed of 50 rpm. To ensure reliability, each specimen underwent the test three times, and the average drilling resistance value from these trials was adopted as the definitive outcome. The test was facilitated by the Sint Technology DRMS 7.0 equipment model.
Microscopic Pore Test
Mercury intrusion test
Targeted block specimens were meticulously collected from regions within 10 mm of the surface, where high salt enrichment was observed, and the pore distribution was tested by mercury intrusion method. The test equipment model is Quantachromre Promaster GT-60.
Salt crystallization characteristics analysis
(1) Ion Chromatography Test. The samples were collected at 1 mm and 10 mm on the surface of the specimen. After the specimen was fully ground, the suspended leaching solution was prepared with deionized water at a water-soil ratio of 50:1, and the leaching solution was shaken and centrifuged. The microporous membrane with a pore size of 0.22 μm was used to filter and extract the supernatant. The anion and cation types and concentrations in the solution were measured by an ion chromatograph. The test equipment model was Thermo Scientific DIONEX ICS-900. (2) Scanning Electron Microscopy and Energy Dispersive Spectrometer (SEM-EDS) test. About 2 cm3 block samples were collected from the surface of the specimen with high salt content within 10 mm. The crystal morphology and position of salt in the specimen were observed by SEM, while the type of salt was confirmed by EDS. The test equipment was JEOL JSM-6610LV.
Results
Deterioration pattern
The morphological characterization of specimens subsequent to undergoing 15 freeze-thaw cycles at varying cooling rates was presented in Fig. 3. It becomes evident that the structural integrity of specimens S, SC-0.33, and SC-0.5 remains relatively preserved, with only minor surface pore enlargement observed. Conversely, SC-0.8 and Cl display fractures approximately 5 mm below the top surface, accompanied by the formation of a loose superficial shell layer. This is manifested by the detachment of fragile powder particles, leading to discernible mass loss and significant structural damage. In essence, an escalating trend in specimen NaCl content corresponds to a more pronounced degree of deterioration. Consequently, a deeper analysis is required to elucidate the degradation mechanisms operative within SC-0.8 and Cl during the freeze-thaw cycling process.
Figure 4 illustrates the morphological alterations observed in SC-0.8 and Cl, respectively, subsequent to 3, 6, 9, 12, and 15 freeze-thaw cycles, each conducted at varying cooling rates. The deterioration patterns exhibited by the specimens follows a consistent progression. Initially, the phenomenon ‘blistering’ manifests on the surface, with the increase of the number of cycles, salt accumulation occurs, leading to the volume expansion, which in turn creates a hollow layer between the surface and the underlying soil. Furthermore, cracks begin to develop and propagate approximately 5 mm beneath the top surface, contributing to a continuous increase in the thickness of the cavity. Ultimately, this process culminates in the complete detachment of the surface shell layer and eventually form flaking and powdering.
Regarding the degree of deterioration, a slower cooling rate precipitates an earlier onset of fracture during the freeze-thaw cycles and culminates more severe degree of deterioration. Notably, after 15 cycles, the surface soil of the specimen subjected to a cooling rate of 0.1 °C/min has completely disintegrated, whereas the shells of specimens cooled at 0.5 °C/min and 1 °C/min remain partially or fully intact, respectively. Additionally, the severity of damage in specimen Cl exceeds that of SC-0.8 under the same conditions, indicating that higher NaCl content exacerbates the deterioration process.
Drilling resistance change
To mitigate the effects of water subjected to freeze-thaw cycling, a statistical comparison was conducted between the mean drilling resistance within discrete depth intervals (0–1, 1–2, 2–3, …, 14–15 mm) from the surface of the specimen and the corresponding mean drilling resistance of a blank specimen at identical depths. This comparison yielded the ratio F/Fb, where Fb represents the drilling resistance of the blank specimen. This ratio, termed as the normalized resistance force (Fn), serves as a quantitative indicator. Specifically, Fn > 1 indicates an increase of soil strength in the salt-containing specimen relative to the blank, whereas Fn < 1 indicates a reduced soil strength in the presence of salt.
The results of the drilling resistance tests are presented in Fig. 5. It can be seen that within 10 mm of the surface layer, the normalized resistance force (Fn) follows the sequence of 0.1 °C/min > 0.5 °C/min > 1 °C/min, indicating that the strength of the surface soil increases as the cooling rate decreases. In other words, slower freezing rates result in a more pronounced increase in the strength of the soil’s surface layer. This phenomenon may be attributed to the formation of larger salt crystals that flocculate with soil particles, fostering the development of aggregates that reinforce soil strength. Within the 10–15 mm subsurface layer, a similar trend is generally observed, albeit with individual specimens exhibiting inconsistent rules, lacking a distinct pattern.
Microstructure characteristics
Figure 6 presents the pore size distribution curve (PSD curve) for S, SC-0.8, and Cl, subsequent to cycling at varying cooling rates. The quantitative assessment of various pore volumes, total porosity, and other parameters are shown in Table 4. The classification of pores adheres to the criteria established by Lei et al, specifically for loess in this region, categorizing them into micro pores (d < 1 μm), small pores (1 μm < d < 4 μm), medium pores (4 μm < d < 16 μm), and large pores (d > 16 μm)30.
The findings indicate minimal variation in pore distribution across different cooling rates, yet notable differences exist in peak value and total porosity. Specifically, the peak value follows the trend of 1 °C/min > 0.1 °C/min > 0.5 °C/min. This phenomenon can be attributed to the fact that at faster cooling rates, salts have limited time to form organized crystal structures, resulting in finer, smaller crystals that failed to effectively fill the pores. Consequently, larger pores dominate the soil structure, consistent with previous findings31. Microstructural analysis further supports this observation, showing smaller crystal sizes in specimens subjected to rapid cooling. Conversely, slower cooling rates promote more extensive salt crystal growth, potentially enhancing pore filling and reducing the occurrence of macropores. In terms of total porosity, the ranking is 0.1 °C/min > 1 °C/min > 0.5 °C/min, suggesting that the slowest cooling rate yields the highest total pore volume. Consequently, among the macroscopic deterioration characteristics examined, the test group with a cooling rate of 0.1 °C/min exhibits the most pronounced deterioration, confirming that slower cooling rates exacerbate the generation of pores and, thus, the degree of deterioration.
To gain deeper insights into variations in pore states, a microstructure state variable \({E}_{m}\) is employed to quantitatively describe the microstructure, following the methodology proposed by Remero et al.32 This variable is defined as Eq. (1):
Herein, \({e}_{m}\) represents the micro-porosity ratio, while \({e}_{M}\) signifies the macro-porosity ratio. The boundary between micro- and macro-porosity is precisely determined by the pore size corresponding to the zero-derivative point on the pore size distribution (PSD) curve. To mitigate the effects of water during the freeze-thaw cycle, the microstructure ratio \({E}_{m}\) of each specimen is calculated based on the micro-pore and macro-pore boundary points of the blank specimen, and the results are shown in Fig. 7. Notably, the microstructure state variables of salt-containing specimens exhibit an increase compared to the blank specimen, indicating a higher proportion of micropores in these samples. This suggests that the presence of salt influences the redistribution of pore structures, favoring the formation of smaller pores.
Salt distribution
To investigate the potential ion differentiation during the freeze-thaw process, particularly between SO4²− and Cl⁻, an analysis was conducted to examine the enrichment patterns of these ions on the surface and within the interior of the specimens. The findings are presented in Fig. 8, in which X0 denotes the initial molar fraction of NaCl within the specimen.
The results show that except for SC-0.8 with a cooling rate of 0.5 °C/min, the NaCl molar fraction in the surface is generally lower than that in the initial state, while the NaCl molar fraction within the interior is obviously increased. This indicates that after 15 freeze-thaw cycles, NaCl tends to accumulate more prominently in the interior, whereas Na2SO4 exhibits a higher concentration on the surface. Furthermore, the proportion of NaCl within the specimens exhibits a distinct trend with varying cooling rates, specifically 1 °C/min > 0.5 °C/min > 0.1 °C/min. This finding underscores that an increase in the cooling rate enhances the migration of Cl− within the soil matrix.
Crystal morphology
To investigate the crystallization morphology of salts in mixed proportions, two kinds of specimen, SC-0.5 and SC-0.8, were selected for detailed analysis. The composition of different salts was determined through energy spectrum analysis, and the results are presented in Figs. 9 and 10, respectively. Notably, Na2SO4 precipitates in a distinctly fine particulate form, occupying the microscopic interstitial spaces among minerals. This morphology diverges from the typical needle- or prism-shaped Na2SO4 crystals observed in evaporation processes33, suggesting that the freeze-thaw cycling promotes recurrent crystallization-dissolution cycles, ultimately eroding the crystalline integrity and resulting in finer particles. Remarkably, the cooling rate has no significant effect on the precipitation morphology of Na2SO4. In contrast, the crystallization behavior of NaCl is significantly affected by the cooling rate. Specifically, the order of crystallization volume follows a descending pattern: 0.1 °C/min > 0.5 °C/min > 1 °C/min. At a cooling rate of 0.1 °C/min, NaCl crystals exhibit a relatively complete structure, encapsulating the surface of particles. At faster cooling rates of 0.5 °C/min and 1 °C/min, the crystals predominantly occupy the tiny pores and exhibit a finer morphology. This observation reveals that slower cooling facilitates more comprehensive crystal growth, resulting in cubic blocks with smooth surfaces that encapsulate the mineral particles. Conversely, rapid cooling hinders proper crystal organization, leading to the formation of fine particles that preferentially occupy pore spaces.
Discussion
Firstly, the crystalline characteristics of Na₂SO₄-NaCl during the cooling process are explained from a theoretical perspective. The crystallization process of pore fluid during cooling occurs in two distinct phases: (1) Initially, as the temperature decreases, the solubility of the salt solution declines, prompting the precipitation of salt within the pores accompanied by volumetric expansion. This phase is referred to as ‘salt expansion’. (2) Subsequently, as cooling continues and the temperature drops further, water within the pores undergoes a transformation into ice, resulting in ‘frost heave’. The deterioration analysis of the blank specimen shows that frost heave caused by water alone produces minimal visible damage. Consequently, the primary cause of deterioration can be attributed to the volumetric expansion associated with salt precipitation.
In Na₂SO₄-NaCl mixed salt solutions, the crystalline phase of Na₂SO₄ significantly impacts the volumetric change during the salt expansion stage. To address this complexity, the phase transitions of salt crystallization during cooling in mixed salt solutions were systematically examined from a crystallographic perspective. According to previous findings of the authors, a suitable thermodynamic model was established based on Pitzer’s ion interaction model to calculate the solubility and water activity of Na₂SO₄-NaCl mixed solutions28. The solubility isotherms across various temperatures were depicted in Fig. 11. During the cooling process, mirabilite remains the stable phase in both pure sodium sulfate solutions and mixed solutions, including S, SC-0.33, and SC-0.5, whereas phase III, thenardite, and heptahydrate manifest as metastable phases. Notably, the equilibrium phase in the SC-0.5 mixture transitions from thenardite to mirabilite. To further validate these theoretical predictions, Raman microscopy and crystal morphology analysis were employed to monitor the crystallization process of solution droplets cooled at a rate of 0.1 °C/min. Our findings, as depicted in Fig. 12, confirm the precipitation of mirabilite in the SC-0.33 and SC-0.8 mixed solutions, thereby supporting the accuracy of the thermodynamic model and enhancing the understanding of phase transitions in such complex salt systems. However, it has been repeatedly established that Na₂SO₄·7H₂O initially precipitates during the cooling of pure sodium sulfate solutions, with a potential transformation to mirabilite under certain conditions34,35. Furthermore, the presence of sodium chloride does not alter the crystallization sequence of sodium sulfate under ambient temperature evaporation28. Therefore, although only mirabilite was observed during the droplet cooling experiments, it is possible that heptahydrate precipitation may have been overlooked due to the rapid supersaturation kinetics of the solution. Regardless of the precipitation process, it is certain that the mixed solution is precipitated in the form of hydrate during the cooling process. However, the low degree of supersaturation and limited crystallization pressure at lower temperatures result in a comparatively low rate of volumetric expansion.
a under 25 °C, b under 10 °C. Solid and dashed curves denote stable and metastable solubilities of mirabilite, thenardite, phase III, heptahydrate and halite, respectively. Red circles give the molalities of Na2SO4 and NaCl in the solutions that are selected for experiments. Green dotted lines represent the relative compositions of these solutions.
The experimental results of this study revealed that specimen S, SC-0.33, and SC-0.5, characterized by high Na₂SO₄ content, exhibited undamaged morphologies, with finely fragmented crystal precipitates consistent with the expansion behavior of Na₂SO₄·7H₂O. Conversely, specimen SC-0.8 and Cl displayed pronounced deterioration, suggesting that under low-temperature conditions, the salt expansion of sodium chloride induces a greater potential for damage compared to the phase transition pressure of sodium sulfate. Furthermore, a slower cooling rate facilitated the migration of Cl− ions within the pore fluid36, enabling the full development of NaCl crystals, which in turn intensified the structural damage to the earth.
Based on the macroscopic, microscopic, and crystalline analyses conducted in this study, and further elaborating on the previous discussion of crystallization behavior in mixed salt solutions, the mechanisms through which different cooling rates affect salt-containing soil deterioration during freeze-thaw cycles are outlined. Considering the crystallization pattern, slower cooling rates promote more substantial crystal formation37, and meanwhile influence the migration of salts. Specifically, for sodium sulfate, faster cooling rates tend to favor surface precipitation. However, surface efflorescence is generally considered beneficial for site preservation, as salt migration and accumulation at the surface result in less damage from crystallization compared to that occurring within the soil pores38. This helps explain why specimens subjected to faster cooling rates experience less severe damage. Taking into account of microstructure, the slowest cooling rate condition results in the highest total porosity, primarily composed of micro- and small pores. In terms of macroscopic deterioration, salt crystals aggregate with soil particles at the surface, forming a crust that marginally enhances soil strength. Nevertheless, this phenomenon ultimately leads to larger-scale flaking and powdering. In summary, these analyses indicate that slower cooling rates exacerbate soil damage, with the deterioration progression unfolding in three stages: blistering, cracking, and flaking.
Some of the experimental results presented in this study are valuable for practical implications for heritage preservation. For instance, for earthen sites located in freeze-thaw regions with high salt content, identifying the types of salts present is crucial for implementing targeted preservation strategies. According to the findings, sodium chloride is likely to cause more severe damage than sodium sulfate in such areas. Therefore, desalination treatments aimed at reducing sodium chloride content might be an effective preservation measure. Furthermore, this study confirms that slower cooling rates result in more extensive deterioration to earthen sites. Therefore, sites with grass-covered surfaces, which experience slower cooling due to thermal insulation in winter, are at higher risk compared to those with exposed surfaces and rapid cooling, when considering only freeze-thaw and salt expansion effects. However, assessing the specific risk level of earthen sites requires consideration of additional factors, such as snowmelt infiltration, erosion, and solar radiation. A more thorough understanding of the internal structural changes in soils and the influence of external conditions under freeze-thaw cycles is necessary for accurate risk assessment. These aspects will be the focus that the authors continue to explore in future study.
In conclusion, this study analyzed the deterioration process of earth containing different proportions of Na2SO4-NaCl mixed salt at different cooling rates through simulation experiments. From the perspective of macroscopic and microscopic deterioration characteristics and salt crystallization characteristics, the influence mechanism of cooling rate on the freeze-thaw deterioration characteristics of salt-containing site earth was discussed. The main conclusions include: (1) The content of NaCl has a significant effect on the freeze-thaw deterioration mode of soil. The specimens S, SC-0.33 and SC-0.5 with low NaCl content have no obvious damage, showing slight structural looseness on the surface. The specimens SC-0.8 and Cl with high NaCl content are loose and cracked on the surface, which leading to flaking and powdering eventually, presenting more sever deterioration. and this structural damage intensifies as the cooling rate decreases. (2) After freeze-thaw cycles, the flocculation of salt and soil particles resulted in the formation of aggregates, leading to an increase in the surface strength of the specimen. Notably, slower cooling rates corresponded to higher surface strength. (3) The cooling rate significantly impacts the distribution ratio of Na₂SO₄ and NaCl. Na₂SO₄ predominantly migrates to the surface and precipitates, whereas NaCl remains more concentrated within the specimen. Higher cooling rate result in a greater proportion of NaCl being retained within the soil. (4) At slower cooling rates, the extended crystal growth period allows for larger and more complete salt crystals. This enhanced crystallization process leads to a decrease in the number of macropores and increases the proportion of micropores, owing to the filling effect of salt crystallization. (5) During the freeze-thaw process, Na₂SO₄ exerts a relatively low crystallization pressure, causing only slight damage to the soil structure. Conversely, when NaCl crystallizes at a slow cooling rate, both the crystal volume and crystallization pressure increase substantially, representing the primary cause of severe structural damage to the soil.
Data availability
The data used in this study are available from the corresponding author upon reasonable request.
References
Sun, M. et al. Classification of deteriorations associated with many earthen heritage sites in arid areas of Northwest China. J. Eng. Geol. 15, 772–778 (2007).
Guo, Q. et al. Key issues and research progress on the deterioration processes and protection technology of earthen sites under multi-field coupling. Coatings 12, 1677 (2022).
Chen, Y. et al. A preliminary study of the freeze-thaw cycle on the structure of earthen sites with different salts. Dunhuang Res. 137, 98–107 (2013).
Li, L. et al. Preservation of earthen heritage sites on the Silk Road, northwest China from the impact of the environment. Environ. Earth Sci. 64, 1625–1639 (2011).
Bing, H. & He, P. Influence of freeze-thaw cycles on physical and mechanical properties of salty soi. Chin. J. Geotech. Eng. 12, 1958–1962 (2009).
Bing, H. & He, P. Experimental investigations on the influence of cyclical freezing and thawing on physical and mechanical properties of saline soil. Environ. Earth Sci. 64, 431–436 (2011).
Pavlík, Z. et al. Computational modelling of coupled water and salt transport in porous materials using diffusion–advection model. J. Frankl. Inst. 348, 1574–1587 (2011).
Kelleners, T. J. et al. Numerical modeling of coupled water flow and heat transport in soil and snow. Soil Sci. Soc. Am. J. 80, 247–263 (2016).
Zhang, J., Lai, Y., Li, J. & Zhao, Y. Study on the influence of hydro-thermal-salt-mechanical interaction in saturated frozen sulfate saline soil based on crystallization kinetics. Int. J. Heat. Mass Transf. 146, 118868 (2020).
Yu, Y. et al. Heat mass transfer and salt frost expansion mechanism of saline soil under freeze-thaw cycle. Journal of Southwest Jiaotong University. 1–8 https://doi.org/10.3969/j.issn.0258-2724.20230299 (2025).
Jiang, J. et al. Study on deformation characteristics of silty sulfate soil under freeze-thaw cycle. Water Resour. Hydropower Eng. 55, 180–190 (2024).
Shen, J. et al. Evolution process of the microstructure of saline soil with different compaction degrees during freeze-thaw cycles. Eng. Geol. 304, 106699 (2022).
Lai, Y., Wu, D. & Zhang, M. Crystallization deformation of a saline soil during freezing and thawing processes. Appl. Therm. Eng. 120, 463–473 (2017).
Lai, Y., Wan, X. & Zhang, M. An experimental study on the influence of cooling rates on salt expansion in sodium sulfate soils. Cold Reg. Sci. Technol. 124, 67–76 (2016).
Wan, X. et al. Water and salt phase change in sodium sulfate soil based on differential scanning calorimetry. Soils Found. 61, 401–415 (2021).
Li, X. et al. Saline expansion and frost heave of sodium sulfate solution during cooling crystallization process. Chin. J. Geotech. Eng. 38, 2069–2077 (2016).
Pu, T., Chen, W., Lv, H. & Du, Y. Analysis on function of deterioration of typical earthen ruins under the coupling of salinized and freezing and thawing in Qinghai−Tibet Plateau. J. Cent. South Univ. 47, 1420–1426 (2016).
Jia, B. et al. Effects of Snowmelt and rainfall infiltration on the water and salt migration of earthen sites during freeze-thaw process. Int. J. Archit. Herit. 17, 573–584 (2023).
Chen, W. et al. Mapping the susceptibility to freeze-thaw deterioration and regionalization of freeze-thaw environments of earthen sites in China: a preliminary study. Sci. Total Environ. 955, 176995 (2024).
Cui, K. et al. The coupling effects of freeze-thaw cycles and salinization due to snowfall on the rammed earth used in historical freeze-thaw cycles relics in northwest China. Cold Reg. Sci. Technol. 160, 288–299 (2019).
Richards, J. et al. Moisture content and material density affects severity of frost damage in earthen heritage. Sci. Total Environ. 819, 153047 (2022).
Chen, W., Jia, B., Tan, Y. & Jia, Q. Freeze-thaw deterioration characteristics of sulfate-contained earthen site soil under snowmelt infiltration. J. Lanzhou Univ. 58, 521–527 (2022).
Chen, W. et al. Freeze-thaw deterioration of saline earthen sites under snowmelt or rainfall infiltration. Chin. J. Geotech. Eng. 44, 334–342 (2022).
Du, Y. et al. Research progress on the development mechanism and exploratory protection of the scaling off on earthen sites in NW China. Sci. China Technol. Sci. 66, 2183–2196 (2023).
Cui, K., Chen, W. & Shen, Y. Experimental study on response of intensity on earthern ruin’s soil undergoing recombination process of salinized and dry-wet in arid and semi-arid regions. J. Cent. South Univ. 43, 4451–4456 (2012).
Zhang, Q. et al. Salt distribution of earthen heritage site wall and its mechanism in northern China. J. Build. Eng. 76, 107154 (2023).
Shen, Y. et al. Effect of salts on earthen materials deterioration after humidity cycling. J. Cent. South Univ. 24, 796–806 (2017).
Shen, Y., Linnow, K. & Steiger, M. Crystallization Behavior and Damage Potential of Na2SO4 –NaCl Mixtures in Porous Building Materials. Cryst. Growth Des. 20, 5974–5985 (2020).
Ministry of Water Resources of the People’s Republic of China. Standard for geotechnical testing method (GB/T 50123-2019). Beijing: China Planning Press (2019).
Lei, X. Pore types and collapsibility of loess in China. Sci. China Ser. B: Chem. 12, 1309–1318 (1987).
Peng, T. & Li, B. The Salt Heave of Sulphate Salty Soil Under Different Cooling Rates. J. Glaciol Geocryol. 19, 252–257 (1997).
Romero, E., Della Vecchia, G. & Jommi, C. An insight into the water retention properties of compacted clayey soils. Géotechnique 61, 313–328 (2011).
Rodriguez-Navarro, C. & Doehne, E. Salt weathering: influence of evaporation rate, supersaturation and crystallization pattern.Earth Surf. Process Landforms 24, 191–209 (1999).
Hamilton, A., Hall, C. & Pel, L. Sodium sulfate heptahydrate: direct observation of crystallization in a porous material. J. Phys. D Appl. Phys. 41, 212002 (2008).
Derluyn, H. et al. Sodium sulfate heptahydrate I: the growth of single crystals. J. Cryst. Growth 329, 44–51 (2011).
Wang, Q. The freezing point of saline loess under freeze-thaw cycle. Master, Xi’an University of Technology Master Thesis (2020).
Wan, X. & Lai, Y. Experimental study on the influence of cooling rate on salth crystallizing in sodium sulphate solution.J. Glacol. Geocryol. 38, 431–437 (2016).
Granneman, S. J. C., Lubelli, B. & Van Hees, R. P. J. Mitigating salt damage in building materials by the use of crystallization modifiers – a review and outlook. J. Cult. Herit. 40, 183–194 (2019).
Ran, Y. & Li, X. Frozen soil map of China (2000). National Tibetan Plateau / Third Pole Environment Data Center. https://doi.org/10.11888/Geocry.tpdc.270552.https://cstr.cn/18406.11.Geocry.tpdc.270552 (2018).
Acknowledgements
This study was funded by Education Department of Shaanxi Provincial Government (Grant No.19JS067).
Author information
Authors and Affiliations
Contributions
C.L.: conceptualization, methodology, experiment and writing; R.L.: experimental analysis, writing and editing; Q.L.: experiment; J.Z.: Experiment; Y.S.: project Leadership, funding acquisition and methodology; M.S.: writing and revision. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liang, C., Liu, R., Li, Q. et al. Study on the influence of cooling rate on the freeze-thaw deterioration characteristics of salt-contaminated earthen site. npj Herit. Sci. 13, 105 (2025). https://doi.org/10.1038/s40494-025-01660-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s40494-025-01660-8