Abstract
Background and aims
Uncorrected obesity contributes to cardiac remodeling and contractile dysfunction although the underlying mechanism remains poorly understood. Mitochondrial aldehyde dehydrogenase (ALDH2) is a mitochondrial enzyme with some promises in a number of cardiovascular diseases. This study was designed to evaluate the impact of ALDH2 on cardiac remodeling and contractile property in high fat diet-induced obesity.
Methods
Wild-type (WT) and ALDH2 transgenic mice were fed low (10% calorie from fat) or high (45% calorie from fat) fat diet for 5 months prior to the assessment of cardiac geometry and function using echocardiography, IonOptix system, Lectin, and Masson Trichrome staining. Western blot analysis was employed to evaluate autophagy, CaM kinase II, PGC-1α, histone H3K9 methyltransferase SUV39H, and Sirt-1.
Results
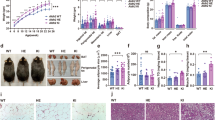
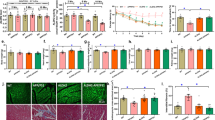
Our data revealed that high fat diet intake promoted weight gain, cardiac remodeling (hypertrophy and interstitial fibrosis, p < 0.0001) and contractile dysfunction (reduced fractional shortening (p < 0.0001), cardiomyocyte function (p < 0.0001), and intracellular Ca2+ handling (p = 0.0346)), mitochondrial injury (elevated O2− levels, suppressed PGC-1α, and enhanced PGC-1α acetylation, p < 0.0001), elevated SUV39H, suppressed Sirt1, autophagy and phosphorylation of AMPK and CaM kinase II, the effects of which were negated by ALDH2 (p ≤ 0.0162). In vitro incubation of the ALDH2 activator Alda-1 rescued against palmitic acid-induced changes in cardiomyocyte function, the effect of which was nullified by the Sirt-1 inhibitor nicotinamide and the CaM kinase II inhibitor KN-93 (p < 0.0001). The SUV39H inhibitor chaetocin mimicked Alda-1-induced protection again palmitic acid (p < 0.0001). Examination in overweight human revealed an inverse correlation between diastolic cardiac function and ALDH2 gene mutation (p < 0.05).
Conclusions
Taken together, these data suggest that ALDH2 serves as an indispensable factor against cardiac anomalies in diet-induced obesity through a mechanism related to autophagy regulation and facilitation of the SUV39H-Sirt1-dependent PGC-1α deacetylation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
27 November 2019
The authors found a critical mistake in the assembly of Fig. 2; in Fig. 2A the right two images were erroneously duplicated. The authors have re-analysed all the data, checked for accuracy and provided the updated Fig. 2 here. Nothing is affected with regards to data summary and conclusion.
References
Emdin CA, et al. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA. 2017;317:626–34. https://doi.org/10.1001/jama.2016.21042
Zhang Y, Ren J. Epigenetics and obesity cardiomyopathy: From pathophysiology to prevention and management. Pharmacol Ther. 2016;161:52–66. https://doi.org/10.1016/j.pharmthera.2016.03.005
DeBoer MD, Gurka MJ. Clinical utility of metabolic syndrome severity scores: considerations for practitioners. Diabetes Metab Syndr Obes. 2017;10:65–72. https://doi.org/10.2147/DMSO.S101624
Lumeng JC. Infant eating behaviors and risk for overweight. JAMA. 2016;316:2036–7. https://doi.org/10.1001/jama.2016.16899
Sackner-Bernstein J, Kanter D, Kaul S. Dietary intervention for overweight and obese adults: comparison of low-carbohydrate and low-fat diets. a meta-analysis. PloS ONE. 2015;10:e0139817. https://doi.org/10.1371/journal.pone.0139817
Raza H, John A, Howarth FC. Alterations in glutathione redox metabolism, oxidative stress, and mitochondrial function in the left ventricle of elderly Zucker diabetic fatty rat heart. Int J Mol Sci. 2012;13:16241–54. https://doi.org/10.3390/ijms131216241
Jia G, Aroor AR, Sowers JR. The role of mineralocorticoid receptor signaling in the cross-talk between adipose tissue and the vascular wall. Cardiovasc Res. 2017;113:1055–63. https://doi.org/10.1093/cvr/cvx097
Carvajal K, et al. Ca(2+) mishandling and cardiac dysfunction in obesity and insulin resistance: role of oxidative stress. Cell Calcium. 2014;56:408–15. https://doi.org/10.1016/j.ceca.2014.08.003
Zhang Y, Ren J. Role of cardiac steatosis and lipotoxicity in obesity cardiomyopathy. Hypertension. 2011;57:148–50. https://doi.org/10.1161/HYPERTENSIONAHA.110.164178
Ozcan L, et al. I. Activation of calcium/calmodulin-dependent protein kinase II in obesity mediates suppression of hepatic insulin signaling. Cell Metab. 2013;18:803–15. https://doi.org/10.1016/j.cmet.2013.10.011
Mathew TS, Ferris RK, Downs RM, Kinsey ST, Baumgarner BL. Caffeine promotes autophagy in skeletal muscle cells by increasing the calcium-dependent activation of AMP-activated protein kinase. Biochem Biophys Res Commun. 2014;453:411–8. https://doi.org/10.1016/j.bbrc.2014.09.094
Pang JJ, Barton LA, Chen YG, Ren J. Mitochondrial aldehyde dehydrogenase in myocardial ischemia-reperfusion injury: from bench to bedside. Sheng Li Xue Bao. 2015;67:535–44.
Ebert AD, et al. Characterization of the molecular mechanisms underlying increased ischemic damage in the aldehyde dehydrogenase 2 genetic polymorphism using a human induced pluripotent stem cell model system. Sci Transl Med. 2014;6:255ra130. https://doi.org/10.1126/scitranslmed.3009027
Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–38. https://doi.org/10.1093/eurheartj/ehq253
Hu N, Zhang Y, Nair S, Culver BW, Ren J. Contribution of ALDH2 polymorphism to alcoholism-associated hypertension. Recent Pat Endocr Metab Immune Drug Discov. 2014;8:180–5.
Ma C, et al. Associations between aldehyde dehydrogenase 2 (ALDH2) rs671 genetic polymorphisms, lifestyles and hypertension risk in Chinese Han people. Sci Rep. 2017;7:11136. https://doi.org/10.1038/s41598-017-11071-w
Yang MY, et al. Activation of aldehyde dehydrogenase 2 slows down the progression of atherosclerosis via attenuation of ER stress and apoptosis in smooth muscle cells. Acta Pharmacol Sin. 2017. https://doi.org/10.1038/aps.2017.81
Pang J, Wang J, Zhang Y, Xu F, Chen Y. Targeting acetaldehyde dehydrogenase 2 (ALDH2) in heart failure—recent insights and perspectives. Biochim Et Biophys Acta. 2017;1863:1933–41. https://doi.org/10.1016/j.bbadis.2016.10.004
Sun A, et al. Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure after myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice. J Am Heart Assoc. 2014;3:e000779. https://doi.org/10.1161/JAHA.113.000779
Yavari A, Ashrafian H. Potentiating mitochondrial aldehyde dehydrogenase 2 to treat post-infarction heart failure. Cardiovasc Res. 2014;103:429–31. https://doi.org/10.1093/cvr/cvu175
Sun A, et al. Aldehyde dehydrogenase 2 ameliorates doxorubicin-induced myocardial dysfunction through detoxification of 4-HNE and suppression of autophagy. J Mol Cell Cardiol. 2014;71:92–104. https://doi.org/10.1016/j.yjmcc.2014.01.002
Zhang Y, Ren J. ALDH2 in alcoholic heart diseases: molecular mechanism and clinical implications. Pharmacol Ther. 2011;132:86–95. https://doi.org/10.1016/j.pharmthera.2011.05.008
Doser TA, et al. Transgenic overexpression of aldehyde dehydrogenase 2 rescues chronic alcohol intake-induced myocardial hypertrophy and contractile dysfunction. Circulation. 2009;119:1941–9. https://doi.org/10.1161/CIRCULATIONAHA.108.823799
Zhang Y, et al. Mitochondrial aldehyde dehydrogenase (ALDH2) protects against streptozotocin-induced diabetic cardiomyopathy: role of GSK3beta and mitochondrial function. BMC Med. 2012;10:40. https://doi.org/10.1186/1741-7015-10-40
Guo Y, et al. A novel protective mechanism for mitochondrial aldehyde dehydrogenase (ALDH2) in type i diabetes-induced cardiac dysfunction: role of AMPK-regulated autophagy. Biochim Et Biophys Acta. 2015;1852:319–31. https://doi.org/10.1016/j.bbadis.2014.05.017
Zhang Y, et al. Mitochondrial aldehyde dehydrogenase 2 accentuates aging-induced cardiac remodeling and contractile dysfunction: role of AMPK, Sirt1, and mitochondrial function. Free Radic Biol Med. 2014;71:208–20. https://doi.org/10.1016/j.freeradbiomed.2014.03.018
Zhang Y, et al. Complex inhibition of autophagy by mitochondrial aldehyde dehydrogenase shortens lifespan and exacerbates cardiac aging. Biochim Et Biophys Acta. 2017;1863:1919–32. https://doi.org/10.1016/j.bbadis.2017.03.016
Wu B, et al. Aldehyde dehydrogenase 2 activation in aged heart improves the autophagy by reducing the carbonyl modification on SIRT1. Oncotarget. 2016;7:2175–88. https://doi.org/10.18632/oncotarget.6814
Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–38. https://doi.org/10.1038/nrm3293
Yang G, et al. The histone H3K9 methyltransferase SUV39H links SIRT1 repression to myocardial infarction. Nat Commun. 2017;8:14941. https://doi.org/10.1038/ncomms14941
Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol Rev. 2014;94:1–34. https://doi.org/10.1152/physrev.00017.2013
Zhang Y, et al. Insulin-like growth factor 1 alleviates high-fat diet-induced myocardial contractile dysfunction: role of insulin signaling and mitochondrial function. Hypertension. 2012;59:680–93. https://doi.org/10.1161/HYPERTENSIONAHA.111.181867
Hu N, Zhang Y. TLR4 knockout attenuated high fat diet-induced cardiac dysfunction via NF-kappaB/JNK-dependent activation of autophagy. Biochim Et Biophys Acta. 2017;1863:2001–11. https://doi.org/10.1016/j.bbadis.2017.01.010
Small L, Brandon AE, Turner N, Cooney GJ. Modelling insulin resistance in rodents by alterations in diet. What have “high fat” and high calorie diets revealed? Am J Physiol Endocrinol Metab. 2017; ajpendo003372017. https://doi.org/10.1152/ajpendo.00337.2017.
Aquilano K, et al. Peroxisome proliferator-activated receptor gamma co-activator 1alpha (PGC-1alpha) and sirtuin 1 (SIRT1) reside in mitochondria: possible direct function in mitochondrial biogenesis. J Biol Chem. 2010;285:21590–9. https://doi.org/10.1074/jbc.M109.070169
Hu N, Ren J, Zhang Y. Mitochondrial aldehyde dehydrogenase obliterates insulin resistance-induced cardiac dysfunction through deacetylation of PGC-1alpha. Oncotarget. 2016;7:76398–414. https://doi.org/10.18632/oncotarget.11977
Matsushima S, Sadoshima J. The role of sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol. 2015;309:H1375–89. https://doi.org/10.1152/ajpheart.00053.2015
Ping Z, et al. The protective effects of salidroside from exhaustive exercise-induced heart injury by enhancing the PGC-1 alpha -NRF1/NRF2 pathway and mitochondrial respiratory function in rats. Oxid Med Cell Longev. 2015;2015:876825. https://doi.org/10.1155/2015/876825
Song MY, Jung HW, Kang SY, Park YK. Atractylenolide III enhances energy metabolism by increasing the SIRT-1 and PGC-1alpha expression with AMPK phosphorylation in C2C12 mouse skeletal muscle cells. Biol Pharm Bull. 2017;40:339–44. https://doi.org/10.1248/bpb.b16-00853
Wang S, Zhu X, Xiong L, Ren J. Ablation of Akt2 prevents paraquat-induced myocardial mitochondrial injury and contractile dysfunction: Role of Nrf2. Toxicol Lett. 2017;269:1–14. https://doi.org/10.1016/j.toxlet.2017.01.009
Roe ND, Ren J. Oxidative activation of Ca(2+)/calmodulin-activated kinase II mediates ER stress-induced cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol. 2013;304:H828–39. https://doi.org/10.1152/ajpheart.00752.2012
Wang S, Zhu X, Xiong L, Zhang Y, Ren J. Toll-like receptor 4 knockout alleviates paraquat-induced cardiomyocyte contractile dysfunction through an autophagy-dependent mechanism. Toxicol Lett. 2016;257:11–22. https://doi.org/10.1016/j.toxlet.2016.05.024
Chen CJ, Fu YC, Yu W, Wang W. SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-kappaB. Biochem Biophys Res Commun. 2013;430:798–803. https://doi.org/10.1016/j.bbrc.2012.11.066
Peng GS, Yin SJ. Effect of the allelic variants of aldehyde dehydrogenase ALDH2*2 and alcohol dehydrogenase ADH1B*2 on blood acetaldehyde concentrations. Hum Genom. 2009;3:121–7.
Yin SJ, Peng GS. Acetaldehyde, polymorphisms and the cardiovascular system. Novartis Found Symp. 2007;285:52–63.
Nishimura FT, et al. Effects of aldehyde dehydrogenase 2 genotype on cardiovascular and endocrine responses to alcohol in young Japanese subjects. Auton Neurosci. 2002;102:60–70.
Li SJ, et al. The high-fat diet induces myocardial fibrosis in the metabolically healthy obese minipigs-The role of ER stress and oxidative stress. Clin Nutr. 2017;36:760–7. https://doi.org/10.1016/j.clnu.2016.06.002
Li L, et al. Mitochondrial biogenesis and PGC-1alpha deacetylation by chronic treadmill exercise: differential response in cardiac and skeletal muscle. Basic Res Cardiol. 2011;106:1221–34. https://doi.org/10.1007/s00395-011-0213-9
Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201–12. https://doi.org/10.1016/j.yjmcc.2008.10.025
Patten IS, Arany Z. PGC-1 coactivators in the cardiovascular system. Trends Endocrinol Metab. 2012;23:90–7. https://doi.org/10.1016/j.tem.2011.09.007
Papanicolaou KN, O’Rourke B, Foster DB. Metabolism leaves its mark on the powerhouse: recent progress in post-translational modifications of lysine in mitochondria. Front Physiol. 2014;5:301. https://doi.org/10.3389/fphys.2014.00301
Dong F, Li Q, Sreejayan N, Nunn JM, Ren J. Metallothionein prevents high-fat diet-induced cardiac contractile dysfunction: role of peroxisome proliferator-activated receptor gamma co-activator 1alpha and mitochondrial biogenesis. Diabetes. 2007;56:2201–12. https://doi.org/10.2337/db06-1596
Huang CY, et al. Adiponectin promotes VEGF-C-dependent lymphangiogenesis by inhibiting miR-27b through a CaMKII/AMPK/p38 signaling pathway in human chondrosarcoma cells. Clin Sci. 2016;130:1523–33. https://doi.org/10.1042/CS20160117
Trivedi PC, et al. Glucolipotoxicity diminishes cardiomyocyte TFEB and inhibits lysosomal autophagy during obesity and diabetes. Biochim Et Biophys Acta. 2016;1861:1893–910. https://doi.org/10.1016/j.bbalip.2016.09.004
Yang Y, et al. Autophagy in cardiac metabolic control: novel mechanisms for cardiovascular disorders. Cell Biol Int. 2016;40:944–54. https://doi.org/10.1002/cbin.10626
Tanaka K, et al. ALDH2 modulates autophagy flux to regulate acetaldehyde-mediated toxicity thresholds. Am J Cancer Res. 2016;6:781–96.
Guo R, Xu X, Babcock SA, Zhang Y, Ren J. Aldehyde dedydrogenase-2 plays a beneficial role in ameliorating chronic alcohol-induced hepatic steatosis and inflammation through regulation of autophagy. J Hepatol. 2015;62:647–56. https://doi.org/10.1016/j.jhep.2014.10.009
Kim JS, Yoon CS, Park DR. NAMPT regulates mitochondria biogenesis via NAD metabolism and calcium binding proteins during skeletal muscle contraction. J Exerc Nutr Biochem. 2014;18:259–66. https://doi.org/10.5717/jenb.2014.18.3.259
Acknowledgements
SW was supported by the University of Wyoming Biomedical Science PhD program.
Funding
American Diabetes Association (7-13-BS-142-BR), NSFC 81370195, NSFC81570225, NSFC81521001, and NSFC 81522004.
Author information
Authors and Affiliations
Contributions
SW, CW, ST, KLR, YZ, data collection; YZ and JR: study design, funding, and manuscript writing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, S., Wang, C., Turdi, S. et al. ALDH2 protects against high fat diet-induced obesity cardiomyopathy and defective autophagy: role of CaM kinase II, histone H3K9 methyltransferase SUV39H, Sirt1, and PGC-1α deacetylation. Int J Obes 42, 1073–1087 (2018). https://doi.org/10.1038/s41366-018-0030-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-018-0030-4
This article is cited by
-
Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) family in physiological and pathophysiological process and diseases
Signal Transduction and Targeted Therapy (2024)
-
ALDH2 mitigates LPS-induced cardiac dysfunction, inflammation, and apoptosis through the cGAS/STING pathway
Molecular Medicine (2023)
-
Stimulating cardiac glucose oxidation lessens the severity of heart failure in aged female mice
Basic Research in Cardiology (2023)
-
A common East-Asian ALDH2 mutation causes metabolic disorders and the therapeutic effect of ALDH2 activators
Nature Communications (2023)
-
Multi-omic analysis of the cardiac cellulome defines a vascular contribution to cardiac diastolic dysfunction in obese female mice
Basic Research in Cardiology (2023)