Abstract
Background/objectives
Survivin is an oncogene associated with a decrease in apoptosis, an increase in tumor growth, and poor clinical outcome of diverse malignancies. A correlation between obesity, cancer, and survivin is reported in the literature. To date, the impact of weight loss on change in survivin levels is understudied. This study was aimed at: (1) comparing survivin levels in adipose tissue (AT) from lean and obese animal models and evaluating changes after weight loss induced by energy restriction and/or exercise; (2) comparing survivin levels in normal weighted and obese humans and evaluating changes in survivin levels after weight loss induced by a very-low-calorie ketogenic diet (VLCKD) or bariatric surgery in AT and/or blood leukocytes (PBL/PBMCs).
Subjects/methods
Survivin expression was evaluated in subcutaneous (SAT) and visceral (VAT) AT derived from animal models of monogenic (Zucker rats) and diet-induced obesity (Sprague Dawley rats and C57BL/6J mice) and after a 4-week weight-loss protocol of energy restriction and/or exercise. Plasma was used to measure the inflammatory status. Survivin expression was also evaluated in PBMCs from patients with obesity and compared with normal weight, in PBLs after VLCKD, and in SAT and/or PBLs after bariatric surgery.
Results
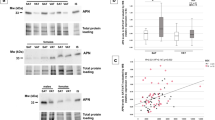
Survivin expression was specifically higher in VAT from obese that lean animals, without differences in SAT. It decreased after weight loss induced by energy restriction and correlated with adiposity and inflammatory markers. In humans, the correlation between being obese and higher levels of survivin was confirmed. In obese subjects, survivin levels were reduced following weight loss after either VLCKD or bariatric surgery. Particularly, a decrease in PBMCs expression (not in SAT one) was found after surgery.
Conclusions
Weight loss is effective in decreasing survivin levels. Also, PBL/PBMC should be regarded as appropriate mirror of survivin levels in VAT for the identification of an obesity-related protumoral microenvironment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22:s176–85.
Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98.
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390:2627–42.
NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–30.
Leitner DR, Fruhbeck G, Yumuk V, Schindler K, Micic D, Woodward E, et al. Obesity and type 2 diabetes: two diseases with a need for combined treatment strategies—EASO can lead the way. Obes Facts. 2017;10:483–92.
Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–80.
Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, et al. Body fatness and cancer-viewpoint of the IARC working group. N Engl J Med. 2016;375:794–8.
Carobbio S, Pellegrinelli V, Vidal-Puig A. Adipose tissue function and expandability as determinants of lipotoxicity and the metabolic syndrome. Adv Exp Med Biol. 2017;960:161–96.
Bluher M. Adipose tissue dysfunction in obesity. Exp Clin Endocrinol Diabetes. 2009;117:241–50.
Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front Endocrinol. 2016;7:30.
Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127:74–82.
Juarez-Rojas JG, Torre-Villalvazo I, Medina-Urrutia AX, Reyes-Barrera J, Sainz-Escarrega VH, Posadas-Romero C, et al. Participation of white adipose tissue dysfunction on circulating HDL cholesterol and HDL particle size in apparently healthy humans. Int J Obes (Lond). 2020;44:920–8.
Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019;20:2358.
Cabia B, Andrade S, Carreira MC, Casanueva FF, Crujeiras AB. A role for novel adipose tissue-secreted factors in obesity-related carcinogenesis. Obes Rev. 2016;17:361–76.
Chadid S, Kreger BE, Singer MR, Loring Bradlee M, Moore LL. Anthropometric measures of body fat and obesity-related cancer risk: sex-specific differences in Framingham Offspring Study adults. Int J Obes (Lond). 2020;44:601–8.
Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–21.
Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol. 2016;29:77–89.
Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5.
Nachmias B, Ashhab Y, Ben-Yehuda D. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin Cancer Biol. 2004;14:231–43.
Als AB, Dyrskjot L, von der Maase H, Koed K, Mansilla F, Toldbod HE, et al. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin Cancer Res. 2007;13:4407–14.
Derin D, Soydinc HO, Guney N, Tas F, Camlica H, Duranyildiz D, et al. Serum levels of apoptosis biomarkers, survivin and TNF-alpha in nonsmall cell lung cancer. Lung Cancer. 2008;59:240–5.
Zaffaroni N, Daidone MG. Survivin expression and resistance to anticancer treatments: perspectives for new therapeutic interventions. Drug Resist Updat. 2002;5:65–72.
Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther. 2006;5:1087–98.
Ejarque M, Ceperuelo-Mallafre V, Serena C, Pachon G, Nunez-Alvarez Y, Terron-Puig M, et al. Survivin, a key player in cancer progression, increases in obesity and protects adipose tissue stem cells from apoptosis. Cell Death Dis. 2017;8:e2802.
Ju L, Zhang X, Deng Y, Han J, Yang J, Chen S, et al. Enhanced expression of Survivin has distinct roles in adipocyte homeostasis. Cell Death Dis. 2017;8:e2533.
Crujeiras AB, Cabia B, Carreira MC, Amil M, Cueva J, Andrade S, et al. Secreted factors derived from obese visceral adipose tissue regulate the expression of breast malignant transformation genes. Int J Obes. 2016;40:514–23.
Izquierdo AG, Carreira MC, Amil M, Mosteiro CS, Garcia-Caballero T, Fernandez-Quintela A, et al. An energy restriction-based weight loss intervention is able to reverse the effects of obesity on the expression of liver tumor-promoting genes. FASEB J. 2019;34:2312–25.
Trimboli P, Castellana M, Bellido D, Casanueva FF. Confusion in the nomenclature of ketogenic diets blurs evidence. Rev Endocr Metab Disord. 2020;21:1–3.
Heiker JT, Kunath A, Kosacka J, Flehmig G, Knigge A, Kern M, et al. Identification of genetic loci associated with different responses to high-fat diet-induced obesity in C57BL/6N and C57BL/6J substrains. Physiol Genomics. 2014;46:377–84.
de Luis D, Domingo JC, Izaola O, Casanueva FF, Bellido D, Sajoux I. Effect of DHA supplementation in a very low-calorie ketogenic diet in the treatment of obesity: a randomized clinical trial. Endocrine. 2016;54:111–22.
Gomez-Arbelaez D, Bellido D, Castro AI, Ordonez-Mayan L, Carreira J, Galban C, et al. Body composition changes after very-low-calorie ketogenic diet in obesity evaluated by 3 standardized methods. J Clin Endocrinol Metab. 2017;102:488–98.
Ortega FJ, Mercader JM, Moreno-Navarrete JM, Nonell L, Puigdecanet E, Rodriquez-Hermosa JI, et al. Surgery-induced weight loss is associated with the downregulation of genes targeted by MicroRNAs in adipose tissue. J Clin Endocrinol Metab. 2015;100:E1467–76.
Dankel SN, Fadnes DJ, Stavrum AK, Stansberg C, Holdhus R, Hoang T, et al. Switch from stress response to homeobox transcription factors in adipose tissue after profound fat loss. PLoS ONE. 2010;5:e11033.
Pinhel MAS, Noronha NY, Nicoletti CF, de Oliveira BAP, Cortes-Oliveira C, Pinhanelli VC, et al. Changes in global transcriptional profiling of women following obesity surgery bypass. Obes Surg. 2018;28:176–86.
Reichert S, Rodel C, Mirsch J, Harter PN, Tomicic MT, Mittelbronn M, et al. Survivin inhibition and DNA double-strand break repair: a molecular mechanism to overcome radioresistance in glioblastoma. Radiother Oncol. 2011;101:51–8.
Vequaud E, Desplanques G, Jezequel P, Juin P, Barille-Nion S. Survivin contributes to DNA repair by homologous recombination in breast cancer cells. Breast Cancer Res Treat. 2016;155:53–63.
Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism. 2019;92:121–35.
Knight BB, Oprea-Ilies GM, Nagalingam A, Yang L, Cohen C, Saxena NK, et al. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells. Endocr Relat Cancer. 2011;18:413–28.
Palianopoulou M, Papanikolaou V, Stefanou N, Tsezou A. The activation of leptin-mediated survivin is limited by the inducible suppressor SOCS-3 in MCF-7 cells. Exp Biol Med. 2011;236:70–6.
Jiang H, Yu J, Guo H, Song H, Chen S. Upregulation of survivin by leptin/STAT3 signaling in MCF-7 cells. Biochem Biophys Res Commun. 2008;368:1–5.
McGaffin KR, Zou B, McTiernan CF, O’Donnell CP. Leptin attenuates cardiac apoptosis after chronic ischaemic injury. Cardiovasc Res. 2009;83:313–24.
Crujeiras AB, Carreira MC, Cabia B, Andrade S, Amil M, Casanueva FF. Leptin resistance in obesity: an epigenetic landscape. Life Sci. 2015;140:57–63.
Izquierdo AG, Crujeiras AB, Casanueva FF, Carreira MC. Leptin, obesity, and leptin resistance: where are we 25 years later? Nutrients. 2019;11:2704.
Chua SC Jr., White DW, Wu-Peng XS, Liu SM, Okada N, Kershaw EE, et al. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr). Diabetes. 1996;45:1141–3.
Crujeiras AB, Gomez-Arbelaez D, Zulet MA, Carreira MC, Sajoux I, de Luis D, et al. Plasma FGF21 levels in obese patients undergoing energy-restricted diets or bariatric surgery: a marker of metabolic stress? Int J Obes. 2017;41:1570–8.
Lopez-Domenech S, Martinez-Herrera M, Abad-Jimenez Z, Morillas C, Escribano-Lopez I, Diaz-Morales N, et al. Dietary weight loss intervention improves subclinical atherosclerosis and oxidative stress markers in leukocytes of obese humans. Int J Obes. 2019;43:2200–9.
Carreira MC, Izquierdo AG, Amil M, Casanueva FF, Crujeiras AB. Oxidative stress induced by excess of adiposity is related to a downregulation of hepatic SIRT6 expression in obese individuals. Oxid Med Cell Longev. 2018;2018:6256052.
Merlotti C, Ceriani V, Morabito A, Pontiroli AE. Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: a critical review and meta-analysis. Int J Obes. 2017;41:672–82.
Acknowledgements
The authors thank Maribel Rendo and Maria Amil from the Department of Molecular and Cellular Endocrinology of the Instituto de Investigacion Sanitaria de Santiago (IDIS) for their support with research data management. This study was supported by the Centro de Investigacion Biomedica en Red de Fisiopatologia de la Obesidad y Nutricion (CIBERobn) and grants from the Instituto de Salud Carlos III-ISCIII (PI17/01287), and it was cofinanced by the European Regional Development Fund (FEDER). ABC is funded by a research contract “Miguel Servet” (CP17/00088) from the ISCIII and cofinanced by the European Regional Development Fund (FEDER).
Author information
Authors and Affiliations
Contributions
AGI and ABC designed the study. AGI, MCC, AFQ, and MPP contributed to the acquisition of the data and samples from the animal models. GRC, MAMO, and AMS contributed to the acquisition of the data and samples from human. GG and DDL performed the nutrition intervention to lose weight. FJO, JMFR, CFN, CBN, and MASP contributed to the acquisition of the data and samples from the bariatric surgery cohort. AGI and ABC performed the statistical analysis. AGI, MCC, and ABC wrote the first draft of the paper, and FFC contributed to the interpretation of data and critical revision of the paper. All authors were involved in the writing of the paper and approved the final version of this article.
Corresponding author
Ethics declarations
Conflict of interest
ABC, FFC, and DDL received research grants and conference fees from Pronokal Spain. GG is medical director of Pronokal Group. The other authors declare no conflict of interest regarding the results of this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Izquierdo, A.G., Carreira, M.C., Rodriguez-Carnero, G. et al. Weight loss normalizes enhanced expression of the oncogene survivin in visceral adipose tissue and blood leukocytes from individuals with obesity. Int J Obes 45, 206–216 (2021). https://doi.org/10.1038/s41366-020-0630-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-020-0630-7
This article is cited by
-
Advancing Obesity Management: the Very Low-Energy Ketogenic therapy (VLEKT) as an Evolution of the “Traditional” Ketogenic Diet
Current Obesity Reports (2025)
-
Nutritional ketosis modulates the methylation of cancer-related genes in patients with obesity and in breast cancer cells
Journal of Physiology and Biochemistry (2025)
-
Mammalian tumor-like organs. 2. Mammalian adipose has many tumor features and obesity is a tumor-like process
Infectious Agents and Cancer (2022)