Abstract
Background
Cardiometabolic diseases are risk factors for COVID-19 severity. The extent that cardiometabolic health represents a modifiable factor to mitigate the short- and long-term consequences from SARS-CoV-2 remains unclear. Our objective was to evaluate the associations between intraindividual variability of cardiometabolic health indicators and COVID-19 related hospitalizations and post-COVID conditions (PCC) among a relatively healthy population.
Methods
This retrospective, multi-site cohort study was a post-hoc analysis among individuals with cardiometabolic health data collected during routine blood donation visits in 24 US states (2009-2018) and who responded to COVID-19 questionnaires (2021–2023). Intraindividual variability of blood pressure (systolic, diastolic), total circulating cholesterol, and body mass index (BMI) were defined as the coefficient of variation (CV) across all available donation timepoints (ranging from 3 to 74); participants were categorized into CV quartiles. Associations were evaluated by multivariable binomial regressions.
Results
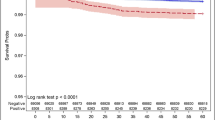
Overall, 3344 participants provided 42,090 donations (median 9 [IQR 5, 17]). The median age was 48 years (38, 56) at the first study donation. 1.2% (N = 40) were hospitalized due to COVID-19 and 15.5% (N = 519) had PCC. Higher BMI variability was associated with greater risk of COVID-19 hospitalization (4th quartile aRR 4.15 [95% CI 1.31, 13.11], p = 0.02; 3rd quartile aRR 3.41 [95% CI 1.09, 10.69], p = 0.04). Participants with higher variability of BMI had greater risk of PCC (4th quartile aRR 1.29 [95% CI 1.02, 1.64]; p = 0.04). Intraindividual variability of blood pressure (systolic, diastolic) and total circulating cholesterol were not associated with COVID-19 hospitalization or PCC risk (all p > 0.05). From causal mediation analysis, the association between the highest quartiles of BMI variability and PCC was not mediated by hospitalization (p > 0.05).
Conclusions
Higher intraindividual variability of BMI was associated with COVID-19 hospitalization and PCC risk. Our findings underscore the need for further elucidating mechanisms that explain these associations and importance for consistent maintenance of body weight.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval.
References
World Health Organization. WHO Coronavirus (COVID-19) dashboard. Geneva, Switzerland: World Health Organization; 2023. https://covid19.who.int/.
Toussi SS, Hammond JL, Gerstenberger BS, Anderson AS. Therapeutics for COVID-19. Nat Microbiol. 2023;8:771–86. https://doi.org/10.1038/s41564-023-01356-4.
El-Sadr WM, Vasan A, El-Mohandes A. Facing the new Covid-19 reality. N Engl J Med. 2023;388:385–7. https://doi.org/10.1056/NEJMp2213920.
Nalbandian A, Desai AD, Wan EY. Post-COVID-19 condition. Annu Rev Med. 2023;74:55–64. https://doi.org/10.1146/annurev-med-043021-030635.
Mueller MR, Ganesh R, Hurt RT, Beckman TJ. Post-COVID conditions. Mayo Clin Proc. 2023;98:1071–8. https://doi.org/10.1016/j.mayocp.2023.04.007.
Centers for Disease Control and Prevention. New ICD-10-CM code for post-COVID conditions, following the 2019 novel coronavirus (COVID-19). 2021. https://www.cdc.gov/nchs/data/icd/announcement-new-icd-code-for-post-covid-condition-april-2022-final.pdf.
Morello R, Mariani F, Mastrantoni L, De Rose C, Zampino G, Munblit D, et al. Risk factors for post-COVID-19 condition (Long Covid) in children: a prospective cohort study. eClinicalMedicine. 2023;59. https://doi.org/10.1016/j.eclinm.2023.101961.
Steenblock C, Schwarz PEH, Ludwig B, Linkermann A, Zimmet P, Kulebyakin K, et al. COVID-19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9:786–98. https://doi.org/10.1016/s2213-8587(21)00244-8.
Stefan N. Metabolic disorders, COVID-19 and vaccine-breakthrough infections. Nat Rev Endocrinol. 2022;18:75–6. https://doi.org/10.1038/s41574-021-00608-9.
Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected—obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17:135–49. https://doi.org/10.1038/s41574-020-00462-1.
Almuwaqqat Z, Hui Q, Liu C, Zhou JJ, Voight BF, Ho Y-L, et al. Long-term body mass index variability and adverse cardiovascular outcomes. JAMA Netw Open. 2024;7:e243062. https://doi.org/10.1001/jamanetworkopen.2024.3062.
Reiterer M, Rajan M, Gómez-Banoy N, Lau JD, Gomez-Escobar LG, Ma L, et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021;33:2174–88.e5. https://doi.org/10.1016/j.cmet.2021.09.009.
Martínez-Colón GJ, Ratnasiri K, Chen H, Jiang S, Zanley E, Rustagi A, et al. SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. Sci Transl Med. 2022;14:eabm9151. https://doi.org/10.1126/scitranslmed.abm9151.
Luo S, Liang Y, Wong THT, Schooling CM, Au Yeung SL. Identifying factors contributing to increased susceptibility to COVID-19 risk: a systematic review of Mendelian randomization studies. Int J Epidemiol. 2022;51:1088–105. https://doi.org/10.1093/ije/dyac076.
Tsampasian V, Elghazaly H, Chattopadhyay R, Debski M, Naing TKP, Garg P, et al. Risk factors associated with post−COVID-19 condition: a systematic review and meta-analysis. JAMA Intern Med. 2023;183:566–80. https://doi.org/10.1001/jamainternmed.2023.0750.
Peluso MJ, Deeks SG. Early clues regarding the pathogenesis of long-COVID. Trends Immunol. 2022;43:268–70. https://doi.org/10.1016/j.it.2022.02.008.
Russell CD, Lone NI, Baillie JK. Comorbidities, multimorbidity and COVID-19. Nat Med. 2023;29:334–43. https://doi.org/10.1038/s41591-022-02156-9.
Lissner L, Odell PM, D’Agostino RB, Stokes J, Kreger BE, Belanger AJ, et al. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–44. https://doi.org/10.1056/nejm199106273242602.
Blair SN, Shaten J, Brownell K, Collins G, Lissner L. Body weight change, all-cause mortality, and cause-specific mortality in the multiple risk factor intervention trial. Ann Intern Med. 1993;119:749–57. https://doi.org/10.7326/0003-4819-119-7_part_2-199310011-00024.
Hussain SM, Newman AB, Beilin LJ, Tonkin AM, Woods RL, Neumann JT, et al. Associations of change in body size with all-cause and cause-specific mortality among healthy older adults. JAMA Netw Open. 2023;6:e237482. https://doi.org/10.1001/jamanetworkopen.2023.7482.
Dasa O, Smith SM, Howard G, Cooper-DeHoff RM, Gong Y, Handberg E, et al. Association of 1-year blood pressure variability with long-term mortality among adults with coronary artery disease: a post hoc analysis of a randomized clinical trial. JAMA Netw Open. 2021;4:e218418. https://doi.org/10.1001/jamanetworkopen.2021.8418.
Wang J, Jin R, Jin X, Wu Z, Zhang H, Han Z, et al. Separate and joint associations of remnant cholesterol accumulation and variability with carotid atherosclerosis: a prospective cohort study. J Am Heart Assoc. 2023;12:e029352. https://doi.org/10.1161/JAHA.122.029352.
Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ. 2016;354:i4098. https://doi.org/10.1136/bmj.i4098.
Schutte AE, Kollias A, Stergiou GS. Blood pressure and its variability: classic and novel measurement techniques. Nat Rev Cardiol. 2022;19:643–54. https://doi.org/10.1038/s41569-022-00690-0.
Sheikh AB, Sobotka PA, Garg I, Dunn JP, Minhas AMK, Shandhi MMH, et al. Blood pressure variability in clinical practice: past, present and the future. J Am Heart Assoc. 2023;12:e029297. https://doi.org/10.1161/JAHA.122.029297.
Clark D III, Nicholls SJ, St John J, Elshazly MB, Ahmed HM, Khraishah H, et al. Visit-to-visit blood pressure variability, coronary atheroma progression, and clinical outcomes. JAMA Cardiol. 2019;4:437–43. https://doi.org/10.1001/jamacardio.2019.0751.
Wang H, Sun X, Von Cannon JL, Kon ND, Ferrario CM, Groban L. Estrogen receptors are linked to angiotensin-converting enzyme 2 (ACE2), ADAM metallopeptidase domain 17 (ADAM-17), and transmembrane protease serine 2 (TMPRSS2) expression in the human atrium: insights into COVID-19. Hypertens Res. 2021;44:882–4.https://doi.org/10.1038/s41440-021-00626-0.
Messerli FH, Rimoldi SF, Bangalore S. Blood pressure variability and arterial stiffness—chicken or egg? JAMA Cardiol. 2019;4:1050. https://doi.org/10.1001/jamacardio.2019.2730.
Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103–18. https://doi.org/10.1016/j.cmet.2014.05.005.
Chavakis T, Alexaki VI, Ferrante AW. Macrophage function in adipose tissue homeostasis and metabolic inflammation. Nat Immunol. 2023;24:757–66. https://doi.org/10.1038/s41590-023-01479-0.
Chaib S, Tchkonia T, Kirkland JL. Cellular senescence and senolytics: the path to the clinic. Nat Med. 2022;28:1556–68. https://doi.org/10.1038/s41591-022-01923-y.
Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–74. https://doi.org/10.1038/s41577-020-0311-8.
Schmitt CA, Tchkonia T, Niedernhofer LJ, Robbins PD, Kirkland JL, Lee S. COVID-19 and cellular senescence. Nat Rev Immunol. 2023;23:251–63. https://doi.org/10.1038/s41577-022-00785-2.
Jones JM, Opsomer JD, Stone M, Benoit T, Ferg RA, Stramer SL, et al. Updated US infection- and vaccine-induced SARS-CoV-2 seroprevalence estimates based on blood donations, July 2020-December 2021. JAMA. 2022;328:298–301. https://doi.org/10.1001/jama.2022.9745.
Jones JM, Stone M, Sulaeman H, Fink RV, Dave H, Levy ME, et al. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA. 2021;326:1400–9. https://doi.org/10.1001/jama.2021.15161.
Department of Health and Human Services, Office of the Assistant Secretary for Health. National research action plan on long COVID. Washington, DC; 2022.
World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. Geneva: World Health Organization; 2000.
Müller SA, Isaaka L, Mumm R, Scheidt-Nave C, Heldt K, Schuster A, et al. Prevalence and risk factors for long COVID and post-COVID-19 condition in Africa: a systematic review. Lancet Glob Health. 2023;11:e1713–24. https://doi.org/10.1016/S2214-109X(23)00384-4.
VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health. 2016;37:17–32. https://doi.org/10.1146/annurev-publhealth-032315-021402.
Geserick M, Vogel M, Gausche R, Lipek T, Spielau U, Keller E, et al. Acceleration of BMI in early childhood and risk of sustained obesity. N Engl J Med. 2018;379:1303–12. https://doi.org/10.1056/NEJMoa1803527.
Sponholtz TR, van den Heuvel ER, Xanthakis V, Vasan RS. Association of variability in body mass index and metabolic health with cardiometabolic disease risk. J Am Heart Assoc. 2019;8:e010793. https://doi.org/10.1161/jaha.118.010793.
Chidambaram V, Shanmugavel Geetha H, Kumar A, Majella MG, Sivakumar RK, Voruganti D, et al. Association of lipid levels with COVID-19 infection, disease severity and mortality: a systematic review and meta-analysis. Front Cardiovasc Med. 2022;9:862999. https://doi.org/10.3389/fcvm.2022.862999.
Mahat RK, Rathore V, Singh N, Singh N, Singh SK, Shah RK, et al. Lipid profile as an indicator of COVID-19 severity: a systematic review and meta-analysis. Clin Nutr ESPEN. 2021;45:91–101. https://doi.org/10.1016/j.clnesp.2021.07.023.
Ketter E, Randall G. Virus impact on lipids and membranes. Annu Rev Virol. 2019;6:319–40. https://doi.org/10.1146/annurev-virology-092818-015748.
Sheppard JP, Nicholson BD, Lee J, McGagh D, Sherlock J, Koshiaris C, et al. Association between blood pressure control and coronavirus disease 2019 outcomes in 45 418 symptomatic patients with hypertension. Hypertension. 2021;77:846–55. https://doi.org/10.1161/HYPERTENSIONAHA.120.16472.
Li FK, An DW, Guo QH, Zhang YQ, Qian JY, Hu WG, et al. Day-by-day blood pressure variability in hospitalized patients with COVID-19. J Clin Hypertens. 2021;23:1675–80. https://doi.org/10.1111/jch.14338.
Jagannatha GNP, Yasmin AAADA, Pradnyana IWAS, Kamardi S, Pradnyaandara IGBMA, Pangkahila EE, et al. Therapeutic target and clinical impact of day-to-day blood pressure variability in hypertensive patients with covid-19. Hypertens Res. 2023;46:165–74. https://doi.org/10.1038/s41440-022-01077-x.
He C, Liu C, Yang J, Tan H, Ding X, Gao X, et al. Prognostic significance of day-by-day in-hospital blood pressure variability in COVID-19 patients with hypertension. J Clin Hypertens. 2022;24:224–33. https://doi.org/10.1111/jch.14437.
Hirten RP, Danieletto M, Tomalin L, Choi KH, Zweig M, Golden E, et al. Use of physiological data from a wearable device to identify SARS-CoV-2 infection and symptoms and predict COVID-19 diagnosis: observational study. J Med Internet Res. 2021;23:e26107. https://doi.org/10.2196/26107.
Natarajan A, Su H-W, Heneghan C. Assessment of physiological signs associated with COVID-19 measured using wearable devices. NPG. Digit Med. 2020;3:156. https://doi.org/10.1038/s41746-020-00363-7.
Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20:442–7. https://doi.org/10.1038/s41577-020-0348-8.
Buttia C, Llanaj E, Raeisi-Dehkordi H, Kastrati L, Amiri M, Meçani R, et al. Prognostic models in COVID-19 infection that predict severity: a systematic review. Eur J Epidemiol. 2023;38:355–72. https://doi.org/10.1007/s10654-023-00973-x.
Thillainadesan S, Madsen S, James DE, Hocking SL. The impact of weight cycling on health outcomes in animal models: a systematic review and meta-analysis. Obes Rev. 2022;23:e13416. https://doi.org/10.1111/obr.13416.
Tschöp MH, Friedman JM. Seeking satiety: from signals to solutions. Sci Transl Med. 2023;15:eadh4453. https://doi.org/10.1126/scitranslmed.adh4453.
Sakers A, De Siqueira MK, Seale P, Villanueva CJ. Adipose-tissue plasticity in health and disease. Cell. 2022;185:419–46. https://doi.org/10.1016/j.cell.2021.12.016.
Carmody RN, Bisanz JE. Roles of the gut microbiome in weight management. Nat Rev Microbiol. 2023;21:535–50. https://doi.org/10.1038/s41579-023-00888-0.
Thaweethai T, Jolley SE, Karlson EW, Levitan EB, Levy B, McComsey GA, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329:1934–46. https://doi.org/10.1001/jama.2023.8823.
Chaichana U, Man KKC, Chen A, Wong ICK, George J, Wilson P, et al. Definition of post–COVID-19 condition among published research studies. JAMA Netw Open. 2023;6:e235856. https://doi.org/10.1001/jamanetworkopen.2023.5856.
Soriano JB, Murthy S, Marshall JC, Relan P, Diaz JV. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–7. https://doi.org/10.1016/S1473-3099(21)00703-9.
National Institute for Health and Care Excellence. COVID-19 rapid guideline: managing the long-term effects of COVID-19. 2021. https://www.nice.org.uk/guidance/ng188.
Altmann DM, Whettlock EM, Liu S, Arachchillage DJ, Boyton RJ. The immunology of long COVID. Nat Rev Immunol. 2023. https://doi.org/10.1038/s41577-023-00904-7.
Imai M, Ito M, Kiso M, Yamayoshi S, Uraki R, Fukushi S, et al. Efficacy of antiviral agents against omicron subvariants BQ.1.1 and XBB. N Engl J Med. 2022;388:89–91. https://doi.org/10.1056/NEJMc2214302.
Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–24. https://doi.org/10.1016/j.ijsu.2014.07.014.
Acknowledgements
We sincerely appreciate all team members of the Repeat Donor Cohort Study including at Vitalant Research Institute, the American Red Cross, Centers for Disease Control and Prevention, and Westat; Dr. Gustaf Edgren for discussion of an earlier iteration of the analysis; and Amber Morris for her assistance with initial exploratory statistics. Figures 1A and 4C were created with BioRender.com. Figure 1B was created with DataWrapper.com.
Funding
This project was supported by the Centers for Disease Control and Prevention (contract number 75D30120C08170) and the National Institute of General Medical Sciences of the National Institutes of Health (R25GM143298 for EAY).
Author information
Authors and Affiliations
Contributions
EAY designed research (project conception, development of overall research plan, and study oversight). MDB, RLB, MPB, and BC conducted research (data collection) and provided essential materials (databases necessary for this study). EAY, MDB, and VIA analyzed data or performed statistical analysis. EAY wrote the initial manuscript draft and had primary responsibility for final content. All authors (EAY, MDB, VIA, RLB, MPB, BC) provided critical feedback and substantive revisions to the manuscript. All authors (EAY, MDB, VIA, RLB, MPB, BC) have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All donors provided informed consent for the use of their deidentified data and residual blood samples from routine blood donations for research as part of voluntarily consenting to donate (Advarra protocol # Pro00030878). The COVID-19 survey was approved by an IRB (Advarra protocol # Pro00056783); all individuals provided informed consent prior to participation. All research involving human subjects conducted at Vitalant conform to the principles contained in the Belmont Report and are subject to the Common Rule and subparts B, C, and D of the US Department of Health and Human Services regulations at 45 CFR part 46. We reported study methodology based on the Strengthening the Reporting of Observational Studies in Epidemiology guidelines [63].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, E.A., Bravo, M.D., Avelino-Silva, V.I. et al. Higher intraindividual variability of body mass index is associated with elevated risk of COVID-19 related hospitalization and post-COVID conditions. Int J Obes 48, 1711–1719 (2024). https://doi.org/10.1038/s41366-024-01603-6
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-024-01603-6