Abstract
Backgrounds
The association between weight change in patients with diabetes, and the development of infective endocarditis (IE) has never been studied. Therefore, we evaluated the associations of weight changes in patients with diabetes with the development of IE.
Methods
In this Korean population-based cohort study, we included patients with diabetes aged ≥20 years who underwent health screenings twice in a 2-year interval between 2009 and 2012. Patients were categorized into five groups according to the degree of weight change between the two health screenings and were followed up until December 2018. A patient with a weight change of ≤-10% was designated to the severe weight loss group, −10 to ≤−5% to the moderate weight loss group, −5 to ≤5% to the stable weight group, 5 to ≤10% to the moderate weight gain group, and ≥10% to the severe weight gain group. The primary outcome was the incidence of IE.
Results
A total of 1,762,108 patients with diabetes were included. There were 67,580 (3.9%) individuals with severe weight loss, 247,969 (14.1%) with moderate weight loss, 1,267,849 (72.0%) with stable weight, 135,774 (7.7%) with moderate weight gain, 42,936 (2.4%) with severe weight gain. During the follow-up (median, 5.21 years), 828 cases of IE occurred. After adjusting for covariates, both weight loss (HR: 2.41, 95% CI: 1.87–3.12 for the severe weight loss group; HR: 1.28, 95% CI: 1.05–1.55 for the moderate weight loss group) and weight gain (HR: 1.17, 95% CI: 0.91–1.50 for the moderate weight gain group; HR: 1.59, 95% CI: 1.11–2.28 for the severe weight gain group) were associated with an increased risk of IE compared to those for the stable weight group.
Conclusion
Both weight gain and weight loss are associated with an increased incidence of IE, and the greater the degree of weight change, the greater the risk.

Similar content being viewed by others
Introduction
Infective endocarditis (IE) is an infection of the endocardial surface of the heart that may involve one or more heart valves [1]. Despite advances in therapeutics, IE remains associated with high morbidity and mortality, resulting in a considerable disease burden [2, 3]. Previous studies have revealed the risk factors for developing IE, including chronic rheumatic heart disease, prosthetic valves, cardiac implantable electronic devices, and hemodialysis [4, 5]. However, studies focusing on risk factors for the development of IE in the general population are lacking.
Diabetes is a known risk factor for the development of IE, independent of renal failure and valve abnormalities [6]. In addition, diabetes could adversely affect mortality and recurrence in patients with IE [7]. In general, weight loss in patients with diabetes is known to improve their metabolic profiles [8, 9]. However, a recent study conducted in a large population showed a U-shaped association between weight change and major cardiovascular events and all-cause mortality [10]. This shows that weight change could have a negative impact on a patient’s health, including the cardiovascular system, and it could be applied to both weight loss and gain.
The association between weight and infectious diseases has been reported [11]. Several studies suggest an increased infection rate in adults with both underweight and obesity [12, 13]. This association between weight and infectious disease was also found in the elderly [14]. Although the association between weight and infectious diseases has been reported, there are very few studies on the relationship between longitudinally observed weight changes and the development of infectious diseases. To the best of our knowledge, the impact of weight change on the incidence of IE is yet to be studied.
Therefore, we aimed to identify the association between weight change in patients with diabetes and the incidence of IE, which has a high disease burden. Through this, we aimed to expand our knowledge on the potential effects of weight change in patients with diabetes.
Methods
Data sources
This study was conducted using data from the National Health Insurance Service - National Health Information Database (NHIS-NHID) of Korea. The data resource profiles of the NHIS-NHID have been published previously [15]. Briefly, the NHIS, which is the single insurer that covers the entire Korean population, is mandatory in Korea and pays costs based on the billing records of healthcare providers. To carry out these processes, the NHIS built a database (NHID) to collect the required information, such as participants’ identifiers, birth date, sex, residence, income levels, and history of diagnoses. Detailed diagnoses were coded using the International Classification of Diseases, Tenth Revision (ICD-10). The NHIS-NHID could be accessed and analyzed for research purposes after receiving approval from the institutional review board (IRB).
This study also used the National Health Insurance Service-Health Screening (NHIS-HEALS) database [16]. In Korea, the NHIS recommends that all insured citizens participate biennially in the health screening program, and the health screening rate was approximately 78.5% in 2017. The NHIS-HEALS database contains data on height, weight, body mass index (BMI), waist circumference (WC), alcohol consumption, smoking habits, blood pressure, fasting blood glucose (FBG), and lipid profiles. Many previous studies have been published using the NHIS-NHID and NHIS-HEALS databases [17,18,19]. The NHIS-NHID and NHIS-HEALS databases were merged in this study. This study was approved by the IRB of Soongsil University (approval no. SSU-202003-HR-201–01). The requirement for informed consent was waived owing to the retrospective nature of the study.
Study population
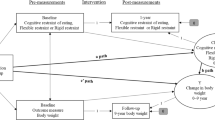
The flow chart of the study population is shown in Fig. 1. First, we identified patients diagnosed with type 2 diabetes during health screening between January 1, 2009 and December 31, 2012. Patients with type 2 diabetes were defined as those who were newly or previously diagnosed with type 2 diabetes during health screening. For information on weight changes, we included adult patients (age ≥20 years) who underwent both the first and second health screening at 2-year intervals before December 31, 2012. We excluded patients with a history of IE before the second health screening. Patients diagnosed with IE or died within 1-year after their second health screening and those with missing data during the health screenings were also excluded. The index date was defined as the date of the second health screening, and baseline characteristics were collected from the index date.
Definitions of variables
Patients with type 2 diabetes were defined as follows: (1) having an ICD-10 code for type 2 diabetes (E11-14) with at least one claim per year for a prescription of anti-diabetic medication or (2) having an FBG ≥ 126 mg/dL in the health screenings without a prescription of oral hypoglycemic agents or insulin [10]. Anti-diabetes medications included metformin, dipeptidyl peptidase 4 inhibitors, sulfonylureas, thiazolidinediones, α-glucosidase inhibitors, meglitinides, and insulin. Anti-diabetes medications were identified in the index year, and the duration of type 2 diabetes was assessed from the date of the first diagnosis of type 2 diabetes to the index date.
Body weight change was defined as the difference in body weight between the first and second health screening. During health screening, a certified examiner recorded anthropometric measurements, including weight (kg). The patients were then categorized into five groups according to the amount of weight change. A patient with a weight change of ≤-10% was designated to the severe weight loss group, −10 to ≤-5% to the moderate weight loss group, −5 to ≤5% to the stable weight group, 5 to ≤10% to the moderate weight gain group, and ≥10% to the severe weight gain group.
Patients with IE were defined as those who were admitted to hospitals with ICD-10 codes (I33.x, I38.x, and I39.8). According to the guidelines, IE requires hospitalization for at least 2 weeks [20, 21], therefore we included those who were hospitalized for more than 14 days or died within 14 days in the analyses; however, those who were hospitalized for ≤14 days and did not die were excluded.
Statistical analysis
Continuous variables are presented as means and standard deviations or medians with interquartile ranges and were compared using the student’s t-test. Categorical variables are presented as frequencies (%) and were compared using the χ2 test. The incidence rate of IE was calculated as the total number of IE cases during the follow-up period divided by 100,000 person-years at risk. The cumulative incidences of IE according to weight changes were plotted, and the differences in the cumulative incidences were compared using the log-rank test. Cox proportional hazard models were used to calculate adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) after adjusting for covariates to identify independent associations between weight changes and IE. Patients with weight changes of −5 to ≤5% were included in the reference group in the multivariable analyses. Subgroup analyses were stratified according to BMI, WC, age, and sex. A two-tailed P < 0.05 was considered statistically significant. All statistical analyses were performed using the SAS software (version 9.4; SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
A total of 1,762,108 individuals with type 2 diabetes were included. The median follow-up duration was 5.21 years (interquartile range, 4.06–6.10 years). There were 67,580 (3.9%) individuals with a weight change of ≤−10%, 247,969 (14.1%) with a weight change of −10 to ≤−5%, 1,267,849 (72.0%) with a weight change of −5% to ≤5%, 135,774 (7.7%) with a weight change of 5 to ≤10%, and 42,936 (2.4%) with a weight change of ≥10%. The weight loss groups tended to be older and had low BMI and WC. The details of the baseline characteristics of each group are shown in Table 1.
U-shaped associations between weight changes and infective endocarditis
During follow-up, 828 cases of IE occurred. The incidence rates of IE were 0.218, 0.106, 0.082, 0.102, and 0.152 for the severe weight loss, moderate weight loss, stable weight, moderate weight gain, and severe weight gain groups, respectively. After adjusting for covariates, both weight loss (HR: 2.41, 95% CI: 1.87–3.12 for the severe weight loss group; HR: 1.28, 95% CI: 1.05–1.55 for the moderate weight loss group) and weight gain (HR: 1.17, 95% CI: 0.91–1.50 for the moderate weight gain group; HR: 1.59, 95% CI: 1.11–2.28 for the severe weight gain group) were associated with an increased risk of IE compared to those for the stable weight group (Table 2). The aHRs of IE incidence according to weight changes showed a U-shaped association, as shown in Fig. 2. The cumulative incidence probabilities for IE between the weight change groups were plotted and showed that both weight loss and weight gain were associated with an increased risk of IE (log-rank P-value < 0.001) (Fig. 3).
Adjusted hazard ratios with 95% confidence intervals are presented after adjusting for covariates (adjusted for age, sex, body mass index, income, smoking, drinking, regular exercise, hypertension, dyslipidemia, chronic kidney disease, fasting blood glucose, insulin use, diabetes mellitus duration, and use of oral hypoglycemic agents). Weight changes of −5 to ≤5% were adopted as the reference group.
Effects of BMI, WC, and other subgroups on the U-shaped associations
To determine whether the effect of weight change on the incidence of IE was modified by baseline BMI status and WC, we stratified the subjects according to BMI and WC. The BMI criterion for classifying the participants into two groups was 25 kg/m2, and the WC criterion was 90 cm for men and 85 cm for women. U-shaped associations between weight changes and the incidence of IE were also identified regardless of the baseline BMI status. Compared with the stable weight group, the severe weight gain group was associated with an increased incidence of IE (HR, 1.85; 95% CI, 1.10–3.11) even when the baseline BMI was <25 kg/m2, and the severe weight loss group was also associated with an increased incidence of IE (HR, 1.89; 95% CI 1.03–3.46) when the baseline BMI was ≥25 kg/m2. This U-shaped association trend was also observed in the WC subgroup; in particular, even in the high WC group, the severe weight loss group was associated with an increased risk of IE (HR, 2.47; 95% CI, 1.57–3.89). In addition to BMI and WC, we performed subgroup analyses by stratifying the participants according to their age and sex. A U-shaped association between weight change and IE incidence was again observed in the subgroup analyses (Fig. 4).
Adjusted hazard ratios with 95% confidence intervals are presented. Weight changes of −5 to ≤5% were adopted as the reference group. Low and high BMIs refer to BMIs <25 kg/m2 and ≥25 kg/m2, respectively. Low WC refers to WC < 90 cm and <85 cm in male and female individuals, respectively. High WC refers to WC ≥ 90 cm and ≥85 cm in male and female individuals, respectively. BMI body mass index, WC waist circumference.
Discussion
This is the first study to analyze the association between weight changes and the incidence of IE using a nationwide, population-based longitudinal follow-up study. As a result, a U-shaped association between weight change and IE was observed in patients with diabetes, regardless of baseline BMI. Compared with the stable weight group, the aHR of IE incidence was the highest in the severe weight loss group.
The reasons for the U-shaped association between weight change and IE could be multifactorial. The incidence of IE increased both in severe weight loss in the high BMI subgroup and severe weight gain in the low BMI subgroup. This indicates that it is not just an effect of losing weight in the underweight group or of gaining weight in the overweight group. A previous study reported U-shaped associations between weight changes and major cardiovascular events, such as heart failure as well as all-cause death in patients with diabetes [10]. Other studies also reported that weight variabilities in patients with diabetes were associated with increased risks of various cardiovascular events [22, 23]. In animal studies, weight variability increased multiple T-helper-1–associated cytokine levels and the number of CD4+ and CD8 + T-cells in adipose tissues [24]. Abdelhafiz et al. reported that weight loss could induce incident hypoglycemic events and frailty in patients with diabetes [25]. Considering all these results, weight changes could affect intimal calcification and contribute to the infection susceptibility of heart valves. Further studies are required to identify the mechanisms underlying this association.
Among the weight change groups, the risk of IE was the highest in the severe weight loss group. Generally, weight loss in patients with diabetes is regarded as beneficial in improving metabolic profiles, especially in patients with obesity [8, 9]. However, some studies have shown that weight loss may increase cardiovascular disease or mortality even in individuals with obesity [10, 26,27,28]. There are several possible explanations for these findings. Previous studies have reported that greater weight loss does not result solely from a reduction in visceral fat, which is critical in metabolic syndrome, but is also associated with decreases in both fat mass and lean mass [29]. The loss of lean mass associated with weight loss can lead to the development of sarcopenia [30], which adversely affects health [31]. Although it is unknown whether the weight change observed in this study was intentional, unintentional weight loss has been documented to negatively affect patient prognosis [32, 33]. In the present study, the proportion of participants who regularly exercised was lower in the weight loss group than in the stable weight group (Table 1). This suggests that the weight loss observed in the weight loss group was more likely to be unintentional than intentional weight reduction through exercise. This suggests that weight loss may have been accompanied by a reduction in lean mass. Therefore, the findings of this study are consistent with those of previous research, suggesting that weight loss may have negative effects on health. The incidence of IE also increased in the weight gain group, which may have been influenced by the higher prevalence of hypertension and chronic kidney disease in this group (Table 1). Previous studies have shown that hypertension is associated with incident IE [34], and chronic kidney disease has been identified as a major risk factor for valvular heart disease [35, 36], which is a risk factor of IE [37]. These factors may have contributed to the higher incidence of IE in the weight gain group than in the stable weight group.
Studies on weight change and the incidence of infectious diseases are limited. Anderin et al. investigated the effects of weight loss before bariatric surgery on the risk of postoperative complications [38]. The participants were prescribed weight-reducing regimens preoperatively. They reported that when patients had a preoperative mean weight loss of −9.5%, the risks of postoperative deep infections or abscesses were significantly reduced (37%) compared to those with stable weight (+ 0.5 kg). However, as the study population was subjected to bariatric surgery and weight loss was achieved by taking a weight-reducing regimen, the results could not explain the general effects of weight change over time on the incidence of infectious disease. Another prospective study was conducted in the US [39]. The study investigated the effects of weight change on community-acquired pneumonia. They reported that the risk of community-acquired pneumonia was nearly two-fold higher among men and women who gained more than 18 kg than among those who maintained their weight during adulthood. Weight loss was not significantly associated with an increased incidence of community-acquired pneumonia. However, the study population was relatively small and information regarding weight was obtained using questionnaires rather than actual measurements. Therefore, to the best of our knowledge, this is the first study to delineate the effect of objectively measured weight change on the incidence of IE in a large study population.
Strengths and limitations
This study had several limitations. First, this was a retrospective observational study, therefore the outcome of IE was defined based on the ICD-10 code, and there might be a risk of inaccuracies. To minimize this, in addition to the ICD-10 code, we defined the length of hospital stay based on the guidelines, and this definition of IE has been validated in previous studies [40, 41]. Second, several variables could not be evaluated because of the inherent limitation of the database; thus, we could not exclude the effects of unmeasured confounders on the results. We could not evaluate the participants’ nutritional habits or the reasons for and intentionality of their weight changes. Additionally, body weight might have changed after the second health screening, but this information was not available. Furthermore, we could not evaluate the etiology of IE and variables that could affect the incidence of IE, such as congenital heart disease or previous valve surgery. Third, participants who did not undergo health screening were not included in this study. Since biennial health screening is required, but not mandatory, it is plausible that patients with relatively good healthcare adherence were mainly included in the study. Nevertheless, considering the relatively high compliance of health screening (78.5%) and the inclusion of more than 1.7 million study participants, we believe that the data is representative of the entire population. Fourth, this study was conducted in South Korea; thus, the results cannot be uniformly applied to other races and countries. Fifth, the study population included patients with diabetes; therefore, the results of this study cannot be generalized to other populations. As weight changes have been associated with increased mortality in both patients with diabetes and the general population [10, 26,27,28], it can be assumed that a similar pattern would be observed in the general population; however, this could not be confirmed in this study. Further studies are warranted to identify whether this association is exclusive to patients with diabetes or is observed in the general population.
Despite these limitations, the main advantage of this study is that long-term follow-up was conducted in a large population. IE is a rare disease, and it is difficult to identify its risk factors in the general population. We were able to identify, for the first time, how general weight changes affect the incidence of IE through the validated definition of IE and long-term follow-up results in a large population. In addition, by performing various subgroup analyses, including subgroups divided according to BMI, it was possible to show that the results were not simply due to the aggravation of cachexia or obesity. This shows that weight change can affect not only metabolic or cardiac outcomes but also the incidence of IE, and has implications for the potential effects of weight change on infectious diseases, which is an area that has not been studied so far. Considering the current disease burden of diabetes and high-risk invasive procedures performed on patients with diabetes, the results of this study are clinically significant.
Conclusions
A U-shaped association was observed between weight change and the incidence of IE. This association was present regardless of the baseline BMI and was most strongly identified in the severe weight loss group. It is worth noting that weight change in patients with diabetes could be a potential risk factor for rare infectious diseases involving the heart.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–30.
Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA. 2018;320:72–83.
Scully PR, Woldman S, Prendergast BD. Infective endocarditis: we could (and should) do better. Heart. 2021;107:96–8.
Şimşek-Yavuz S, Şensoy A, Kaşıkçıoğlu H, Ceken S, Deniz D, Yavuz A, et al. Infective endocarditis in Turkey: aetiology, clinical features, and analysis of risk factors for mortality in 325 cases. Int J Infect Dis. 2015;30:106–14.
Chaudry MS, Carlson N, Gislason GH, Kamper A-L, Rix M, Fowler Jr VG, et al. Risk of infective endocarditis in patients with end stage renal disease. Clin J Am Soc Nephrol CJASN. 2017;12:1814.
Movahed MR, Hashemzadeh M, Jamal MM. Increased prevalence of infectious endocarditis in patients with type II diabetes mellitus. J Diab Complicat. 2007;21:403–6.
Yoshioka D, Toda K, Yokoyama J-y, Matsuura R, Miyagawa S, Kainuma S, et al. Diabetes mellitus adversely affects mortality and recurrence after valve surgery for infective endocarditis. J Thorac Cardiovasc Surg. 2018;155:1021–9.e5.
Henry R, Wallace P, Olefsky J. Effects of weight loss on mechanisms of hyperglycemia in obese non-insulin-dependent diabetes mellitus. Diabetes. 1986;35:990–8.
Rothberg AE, McEwen LN, Kraftson AT, Ajluni N, Fowler CE, Nay CK, et al. Impact of weight loss on waist circumference and the components of the metabolic syndrome. BMJ Open Diab Res Care. 2017;5:e000341.
Park CS, Choi Y-J, Rhee T-M, Lee HJ, Lee H-S, Park J-B, et al. U-Shaped Associations between body weight changes and major cardiovascular events in type 2 diabetes mellitus: a longitudinal follow-up study of a nationwide cohort of over 1.5 million. Diab Care. 2022;45:1239–46.
Dobner J, Kaser S. Body mass index and the risk of infection-from underweight to obesity. Clin Microbiol Infect. 2018;24:24–8.
Phung DT, Wang Z, Rutherford S, Huang C, Chu C. Body mass index and risk of pneumonia: a systematic review and meta-analysis. Obes Rev. 2013;14:839–57.
Harpsøe MC, Nielsen NM, Friis-Møller N, Andersson M, Wohlfahrt J, Linneberg A, et al. Body mass index and risk of infections among women in the danish national birth cohort. Am J Epidemiol. 2016;183:1008–17.
Dorner TE, Schwarz F, Kranz A, Freidl W, Rieder A, Gisinger C. Body mass index and the risk of infections in institutionalised geriatric patients. Br J Nutr. 2010;103:1830–5.
Cheol Seong S, Kim Y-Y, Khang Y-H, Heon Park J, Kang H-J, Lee H, et al. Data resource profile: the national health information database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800.
Seong SC, Kim Y-Y, Park SK, Khang YH, Kim HC, Park JH, et al. Cohort profile: the national health insurance service-national health screening cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7:e016640.
Lee S-R, Choi E-K, Jung J-H, Han K-D, Oh S, Lip GY. Lower risk of stroke after alcohol abstinence in patients with incident atrial fibrillation: a nationwide population-based cohort study. Eur Heart J. 2021;42:4759–68.
Kim MK, Han K, Park Y-M, Kwon H-S, Kang G, Yoon K-H, et al. Associations of variability in blood pressure, glucose and cholesterol concentrations, and body mass index with mortality and cardiovascular outcomes in the general population. Circulation. 2018;138:2627–37.
Kang S-H, Choi E-K, Han K-D, Lee S-R, Lim W-H, Cha M-J, et al. Underweight is a risk factor for atrial fibrillation: A nationwide population-based study. Int J Cardiol. 2016;215:449–56.
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta J-P, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–128.
Baddour LM, Wilson WR, Bayer AS, Fowler Jr VG, Tleyjeh IM, Rybak MJ, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132:1435–86.
Nam GE, Kim W, Han K, Lee C-w, Kwon Y, Han B, et al. Body weight variability and the risk of cardiovascular outcomes and mortality in patients with type 2 diabetes: a nationwide cohort study. Diab Care. 2020;43:2234–41.
Lee H-J, Choi E-K, Han K-D, Kim DH, Lee E, Lee S-R, et al. High variability in bodyweight is associated with an increased risk of atrial fibrillation in patients with type 2 diabetes mellitus: a nationwide cohort study. Cardiovasc Diabetol. 2020;19:1–10.
Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–8.
Abdelhafiz AH, McNicholas E, Sinclair AJ. Hypoglycemia, frailty and dementia in older people with diabetes: reciprocal relations and clinical implications. J Diab Complicat. 2016;30:1548–54.
Kwon SY, Kim G, Lee J, Park J, Lee Y-B, Jin S-M, et al. Association of body weight change with all-cause and cause-specific mortality: A nationwide population-based study. Diab Res Clin Pract. 2023;199:110666.
Huang Q-M, Shen D, Gao J, Chen H, Xie J-H, Yan H-Y, et al. Association of weight change with all-cause and cause-specific mortality: an age-stratified analysis. BMC Med. 2024;22:438.
Kim MK, Han K, Koh ES, Kim ES, Lee M-K, Nam GE, et al. Weight change and mortality and cardiovascular outcomes in patients with new-onset diabetes mellitus: a nationwide cohort study. Cardiovasc Diabetol. 2019;18:1–12.
Chaston TB, Dixon J. Factors associated with percent change in visceral versus subcutaneous abdominal fat during weight loss: findings from a systematic review. Int J Obes. 2008;32:619–28.
Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12:487–91.
Kitamura A, Seino S, Abe T, Nofuji Y, Yokoyama Y, Amano H, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. 2021;12:30–8.
De Stefani FdC, Pietraroia PS, Fernandes-Silva MM, Faria-Neto J, Baena CP. Observational evidence for unintentional weight loss in all-cause mortality and major cardiovascular events: a systematic review and meta-analysis. Sci Rep. 2018;8:15447.
Bosch X, Monclús E, Escoda O, Guerra-García M, Moreno P, Guasch N, et al. Unintentional weight loss: Clinical characteristics and outcomes in a prospective cohort of 2677 patients. PloS one. 2017;12:e0175125.
Lee GB, Shin KE, Han K, Son H-S, Jung J-S, Kim Y-H, et al. Association between hypertension and incident infective endocarditis. Hypertension. 2022;79:1466–74.
Marwick TH, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, et al. Chronic kidney disease and valvular heart disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019;96:836–49.
Ureña-Torres P, D’Marco L, Raggi P, García–Moll X, Brandenburg V, Mazzaferro S, et al. Valvular heart disease and calcification in CKD: more common than appreciated. Nephrol Dialysis Transplant. 2020;35:2046–53.
Østergaard L, Valeur N, Wang A, Bundgaard H, Aslam M, Gislason G, et al. Incidence of infective endocarditis in patients considered at moderate risk. Eur Heart J. 2019;40:1355–61.
Anderin C, Gustafsson UO, Heijbel N, Thorell A. Weight loss before bariatric surgery and postoperative complications: data from the Scandinavian obesity registry (SOReg). Ann Surg. 2015;261:909–13.
Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–8.
Tan C, Hansen M, Cohen G, Boyle K, Daneman N, Adhikari NK. Accuracy of administrative data for identification of patients with infective endocarditis. Int J Cardiol. 2016;224:162–4.
Lee GB, Shin KE, Han K, Son HS, Jung JS, Kim YH, et al. Association between hypertension and incident infective endocarditis. Hypertension. 2022. https://doi.org/10.1161/HYPERTENSIONAHA.122.19185.
Acknowledgements
We appreciate the Medical Illustration & Design (MID) team, a member of Medical Research Support Services of Yonsei University College of Medicine, for their excellent support with medical illustration. We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (No. 2022R1C1C1010012) and a faculty research grant of Yonsei University College of Medicine (6-2023-0084).
Author information
Authors and Affiliations
Contributions
J.H.K. and S.H.P. drafted the manuscript. J.H.K., S.H.P., K.H., S.H.L. and N.S.K. contributed to the conception, design, analysis, and interpretation of the data. K.H. contributed to the acquisition of data. J.H.K., S.H.P., S.J.L., J.K., W.K.P., H.K., J.Y.A., S.J.J., J.Y.C., J.Y., K.H., S.H.L. and N.S.K. contributed to the discussion and critically revised the manuscript. All the authors have read and approved the final manuscript and have agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the institutional review board of Soongsil University (approval no. SSU-202003-HR-201–01). The requirement for informed consent was waived owing to the retrospective nature of the study. All methods in this study were performed in accordance with the relevant guidelines and regulations.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kim, J.H., Park, S.H., Lee, S.J. et al. Association between weight changes and infective endocarditis in patients with diabetes: A nationwide population-based cohort study. Int J Obes 49, 658–664 (2025). https://doi.org/10.1038/s41366-024-01687-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-024-01687-0