Abstract
Background
Cost-effective and easy-to-implement nutritional approaches to overcoming obesity and accompanying diseases remain of considerable therapeutic interest. Collagen peptides (CP) have previously demonstrated potential to alleviate obesity and related symptoms during high-fat/ high-caloric diets. Therefore, a systematic review and meta-analysis have been conducted to pool the evidence from the last decades.
Methods
A systematic literature search was conducted in accordance with the PRISMA statement in PubMed, Scopus, CINAHL, MEDLINE and ScienceDirect in March 2024. Risk of bias was assessed using the SYRCLE tool for animal studies. A random-effects model with standardized mean differences (SMD) calculated overall effect sizes. Preclinical controlled trials as well as randomized controlled trials were eligible if rodents received a high-fat or high-caloric diet, were genetically not modified and administered CPs compared to a placebo for at least 3 weeks.
Results
Twenty-one and nineteen (n = 339) studies were included in the systematic review and meta-analysis, respectively. A several-week CP supplementation led to significant decreases in body mass (p < 0.01; SMD = −1.87), food intake (p = 0.01; SMD = −1.43), glucose metabolism (p < 0.02; SMD = −2.21), adipose tissue & organ content (p < 0.01; SMD = −1.26), LDL (p < 0.01; SMD = −2.02), triacylglycerol (p < 0.01; SMD = −2.16) and leptin (p = 0.01; SMD = −1.33). Significant increases were observed in HDL (p = 0.04; SMD = 1.14) and adiponectin (p < 0.01; SMD = 1.04). A moderate to high risk of bias, combined with a very low certainty of evidence for each parameter, was apparent.
Conclusion
CP administration in rodents with obesity for at least 3 weeks demonstrated significant anti-obesity effects by positively influencing body mass, food intake, glucose metabolism, lipid markers, adipose tissue and adipokines. However, CP’s anti-obesity effects in humans remain largely unexplored, necessitating further research.
Similar content being viewed by others
Introduction
According to the World Obesity Federation, ~40% of the global population currently faces overweight or obesity, predicting to reach a yet unprecedented milestone of over 50% in 2035 [1]. If the trend of increasing obesity rates persists, it is anticipated that there will be significant economic costs amounting to approximately 3.3% of the global gross domestic product by 2060 [2]. Obesity, expressed as an elevated body mass index above 29.9, increases the risk of non-communicable diseases such as diabetes mellitus type II (DMT2), cardiovascular diseases and musculoskeletal disorders, leading to a significant decline in both quality of life and life expectancy. As the current rationale for obesity constitutes an excessive caloric intake leading to an overall energy imbalance/surplus, the pathophysiology appears to be of a multifactorial nature [3]. Mechanisms responsible for overfeeding, ectopic lipid accumulation, brain-associated reward regulation, as well as social and physical factors are presently of research interest. Moreover, little is known about genetic and epigenetic causes and the influence on and of the gut microbiome, which may play a pivotal role in obesity [4]. Approaches to adjust individual lifestyles by means of nutritional modifications have been demonstrated to effectively aid in obesity management [5]. Both the amount and source of proteins and even their inherent peptides could be viewed as a prospective therapeutic strategy for influencing body composition, markers of metabolic syndrome and appetite regulation in patients with obesity [6].
Of late, collagen peptides (CP), being unique in their amino acid composition (mainly comprised of glycine, proline, hydroxyproline) and usually received from connective tissues of marine or mammalian sources, have elicited interest in obesity management research. The majority of studies conducted as animal trials have addressed the potential alleviation of metabolic dysregulation associated with high-caloric diet (HCD)-fed rodents in addition with CP administration [7]. Absolute body weight over time compared to a HCD control group has been reported to be lower in Wistar rats supplemented with fish CPs for 2 months, while food intake remained the same [8]. A study applying CPs for 9 weeks, alongside additional fish-derived extracts, demonstrated improved glucose sensitivity during a glucose tolerance test, slight changes in relative abdominal fat deposition, and reduced leptin mRNA expression levels compared to a control high-fat diet (HFD). These findings suggest that CPs may contribute to improved hunger-satiety regulation, help combat leptin resistance in organisms facing obesity, and enhance insulin sensitivity [9]. Lipid serum markers such as total and low-density lipoprotein (LDL) cholesterol and triacylglycerol, which are commonly elevated (= dyslipidemia) in metabolic syndrome and patients with obesity [10], also being predictors of cardiovascular diseases [11], have been reported to be lower in fish CP + HFD-fed mice after 8 weeks [12, 13].
Some of the yet investigated molecular mechanisms involved in obesity include transcription factors that strictly control energy expenditure and storage. Peroxisome proliferator-activated receptor gamma (PPARγ), recognized as one master regulator of adipogenesis, and CCAAT-enhancer-binding proteins (C/EBPs) regulate differentiation of preadipocytes and mesenchymal stem cells to mature adipocytes, based on physiological lipid concentrations. Another transcription factor, Sterol regulatory element-binding proteins (SREBPs), enhances transcription of genes responsible for triglyceride and fatty acid synthesis, which is induced by insulin and therefore also hinged on glucose metabolism [14]. Gene expression levels of PPARγ and C/EBPs have previously been shown to decrease in mouse 3T3-L1 pre-adipocytes following CP addition for 8 days [12]. Likewise, hepatic protein expression of the isoforms SREBP-1 and SREBP-2 (responsible for cholesterol synthesis) was both significantly reduced in C57BL6/J male mice after an 8-week CP administration, conveying a trend towards decreased levels with increased dosages [15].
Obesity induced systemic inflammation has been reported to promote insulin resistance and DMT2-associated complications such as retinopathy and cardiovascular disease [16]. Thus, therapeutic approaches to reduce obesity related inflammatory states are crucial to halt the development of concomitant diseases. One of numerous anti-inflammatory proteins illustrates the insulin-sensitizing adiponectin that hinders maturation of pre-adipocytes to adipocytes [17]. Together with common pro-inflammatory proteins like tumor necrosis factor alpha (TNFα) and C-reactive protein (CRP), the addition of high-dose marine CPs for 1 month revealed positive effects represented by declines in TNFα & CRP and increases in adiponectin expression in type II diabetic rats [18]. Strong anti-inflammatory activity was also observed in apolipoprotein E-deficient mice by two specific CPs derived from salmo sala collagen (~1.2 kDa) by means of a nano-liquid chromatography–tandem mass spectrometry, depicting probable atherosclerosis inhibition effects [19]. Since inflammation often appears to be the cause of augmented oxidative stress due to an upregulation of genes involved in inflammatory pathways [20], CPs have also been reported to deliver antioxidant activity. In obesity-induced db/db mice, an 8-week skate CP supplementation intervention led to reduced concentrations of oxidative stress-related markers and increased the concentration of antioxidants, thereby reducing obesity-induced oxidative stress [21]. Similar results were also obtained in a linoleic acid model system, demonstrating autooxidation inhibition and effective scavenging of radicals [22]. CPs from milkfish even protected against UV radiation and H2O2-induced DNA single-strand breaks in an in vitro model of human keratinocytes [23].
Although of a correlational nature, recent evidence suggests that the gut microbiome majorly influences energy homeostasis and body mass regulation. HFDs and high-sucrose diets have been shown to induce negative alterations in specific gut microbiota, such as Firmicutes to Bacteroidetes, where a higher ratio (F/B) of these is usually found in subjects with obesity [24]. In a study administering two CPs from different marine sources over 2 weeks to Sprague Dawley rats, the F/B ratio remained either equal or got lower compared to a normal non-HFD group [25]. In contrast, an increase in the F/B ratio has been reported in CP-receiving mice following a 12-week HFD [26]. Based on these ambiguous findings, more evidence is needed to clarify the interplay between specific diets and the host’s microbial composition.
Therefore and according to the previously mentioned issues, this systematic review and meta-analysis investigates the potential anti-obesity impact of CP supplementation on body mass, food intake, lipid and glucose metabolism, antioxidant capacity, anti-inflammatory status and the gut microbiome of rodents.
Methods
The current systematic review and meta-analysis were registered in advance on the Open Science Framework (Registration: https://doi.org/10.17605/OSF.IO/38G7F) and developed following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA).
Eligibility criteria
Experimental preclinical trials investigating mice and rats with exercise interventions lasting more (or equal) than 3 weeks were considered for inclusion. Studies conducting trials with genetically modified/ knock-out rodents (e.g., ApoE-/-), in vitro and ex vivo models were excluded. Since anti-obesity-related effects were of interest in the current review, both the treatment and the control group had to follow a high-calorie or high-fat diet. Regardless of its origin and manufacturer, the treatment group additionally received CP regularly. Alcohol, streptozotocin (to induce a T2DM model), additional vitamins, amino acids or other potentially confounding bioactive peptides were prohibited. If studies were published in other languages than English, ChatGPT was used for translation to check for suitability. Moreover, animal- preclinical controlled trials as well as randomized controlled trials were eligible for inclusion, whereas case control and observational studies were excluded.
Search strategy
Comprehensive literature search was performed in April 2024 using PubMed, EBSCOhost (CINAHL, MEDLINE), Scopus and ScienceDirect. The search strings were as follows: collagen peptide* AND (supplementation* OR administration* OR effect* OR intake OR consumption) AND (“anti-obesity” OR “anti obesity” OR obesity OR adiposity OR adipose OR obese OR fat) AND (animal* OR rodent* OR mice OR mouse OR rodentia OR rat*) AND (“high-fat diet” OR “high fat diet” OR “high caloric diet” OR “high calori diet” OR “high-caloric diet” OR “high-calori diet”). An additional filter in Scopus was used (“Article title, Abstract, Keywords” instead of “All fields”). Due to a restriction to a maximum of eight boolean operators, an adapted search string was applied for ScienceDirect: collagen peptide AND (“anti-obesity” OR “anti obesity”) AND (rodent OR mice OR rat OR animal) AND (high fat diet OR high calori). We conducted a screening of references from the included studies and also utilized Google Scholar for both forward and backward searches. If studies met the eligibility criteria during this process, they were “handpicked” and eligible for inclusion. While gray literature was not actively pursued, it was taken into consideration if it met the eligibility criteria. Following the initial search string query across all databases, literature screening was independently conducted by two reviewers (AMM, KB) using the free online Rayyan tool (https://www.rayyan.ai/). Any discrepancies in the inclusion or exclusion of articles during title, abstract, and full-text screening were resolved through consensus between the two reviewers. If consensus could not be reached, a third reviewer (DK) made the final decision. Data from included studies were primarily collected by KB directly from the articles or through requests to the authors.
Data collection
The number of animals per group, study design, strain, age, dose, duration of supplementation phase, control diet, origin of CP and outcome parameters were extracted from the included studies and are presented in Table S1. Since several studies included more than a single treatment group, all dosages were listed, but only the highest dose of each trial was considered for meta-analysis. If the required data were not available within the articles, corresponding authors were contacted via email and/or Researchgate to request the information. If authors did not responded, mean and standard deviation (SD) values were extracted from graphs using WebPlotDigitizer (https://automeris.io/WebPlotDigitizer/). If only standard error (SE) instead of SD was provided, SD was calculated using the formula \(\mathrm{SD}=\mathrm{SE}* \sqrt{N}\) [27]. For the meta-analysis, individual parameters for each study were selected for the following items. Body mass, food/energy intake, adipocyte size (adipocyte diameter and cross-sectional area), adipose tissue and organ content (dorsal fat, subcutaneous (white) adipose tissue (WAT), epididymal WAT, visceral adipose tissue, liver and liver index (weight/body weight in %)), glucose metabolism (glucose, insulin, HbA1c), serum lipids (total cholesterol, LDL, HDL, triacylglycerol), oxidative stress (TBARS, SOD, GPx, CAT, GSH, MDA, ROS), inflammation (TNFα, IL1β, IL4, IL6, IL10, IL12, NFκB), adipokines (adiponectin, leptin) and lipid metabolism related transcription factors (PPARα, PPARγ, SREBP1 & 2, C/EBPα). Regarding gut microbiome (firmicutes/ bacteroidetes ratio), one out of three relevant trials depicted mean ± SD, for the remaining two, only the average without SD has been obtained. Due to the low number of data, the gut microbiome did not enter the meta-analysis, but was included in the systematic review.
Risk of bias and certainty of evidence assessment
To assess the risk of bias in preclinical trials including animals, the SYstematic Review Centre for Laboratory animal Experimentation (SYRCLE) tool was utilized as an adapted version of the Cochrane RoB tool, which is usually applied in systematic reviews of human RCTs [28]. The SYRCLE tool examines biases related to selection, performance, detection, attrition, reporting and other sources of bias. In total, 10 items are represented and each can be rated by “yes”, “unclear” or “no”, where a rating high in “yes” scores is indicative of low risk of overall bias. As recommended by the authors [28], a total score for each study was not calculated. Rather, an individual approach was taken, where overall differences and similarities among studies were inspected and interpreted. The methodological evaluation of the included studies was conducted by AMM and KB, with any disagreements resolved by a third reviewer, DK. Moreover, publication bias risk in parameters containing more than ten studies/data sets was assessed using contour-enhanced funnel plots and Egger’s test. The certainty of evidence was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) [29]. Given that all the studies included were randomized controlled trials (RCTs) or at least controlled trials in a parallel group design manner, the grading initially began at a level of “high” certainty. However, outcomes had the potential to be downgraded to “very low” certainty due to various factors such as risk of bias, inconsistency, indirectness, imprecision, and publication bias. In addition, an upgrade was not possible within these study types.
Meta-analysis
Meta-analyses have been conducted in R (version 4.2.3) using the “metafor” (v. 4.8-0) and “meta” (v. 8.0-2) packages. The analyses utilized the standardized mean difference (SMD), including change scores (post–pre values, resulting in Δmean and ΔSD) and post values gauged in different units/scales since several authors did not respond to data requests. A random-effects model was applied across all variables, with effect sizes expressed as SMD (Hedges’ g) and a 95% confidence interval, illustrated by means of forest plots. Multilevel meta-analysis has been carried out (with “metafor”) for “glucose metabolism”, “inflammation”, “oxidative capacity”, “adipose tissue & organ content” and “lipid metabolism related transcription factors” since studies with multiple variables were included, and therefore, independency between estimators was not given anymore. For all other parameters, a conventional meta-analysis has been applied (with “meta”). The restricted maximum-likelihood was chosen as the model estimator. Heterogeneity was assessed through I² (where 25%, 50%, and 75% are considered low, moderate, and high variance, respectively) [30] and Cochran’s Q-statistic (with p < 0.05 indicating studies do not share a common effect size [31]). If biomarkers in a specific analysis exhibit opposite beneficial effects (e.g., low ROS and high SOD indicating enhanced antioxidant capacity), signs have been reversed (+ to – and vice versa). The individual changes can be found in the descriptions of the relevant forest plots. Publication bias is usually checked if 10+ studies are involved in a meta-analysis, but we checked all parameters by visually inspecting contour-enhanced funnel plots (Supplementary file 1) and by calculating the p-values of Egger’s regression test (p < 0.05 implying potential publication bias). Subgroup analysis was performed to explore origins of heterogeneity for body mass due to having included the most studies (n = 18) of all parameters. Factors examined were species (rats/mice), intervention duration (≤ 6 weeks, > 6 weeks), age (< 8 weeks, ≥ 8 weeks; since rats are considered adult after approximately 8 weeks [32]) and CP source (marine/mammals).
Results
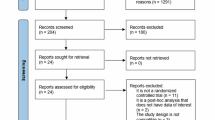
Out of 374 articles obtained from the initial search, 18 were assessed for eligibility (Fig. S1). Three of them were excluded due to not providing the full text, inappropriate diet design [8] (control and treatment group were additionally substituted with alcohol) and genetically modified (apolipoprotein E-deficient (ApoE-/-)) mice [19]. Identification of studies via other methods (websites (Google Scholar) and citation searching, e.g., citation mining, forward and backward citation search) led to eleven articles from which five were excluded because of genetic modification (db/db rats), streptozotocin-induced type 2 diabetes mellitus, no full-text provision, CP supplementation mixed with cod(-fish) powder and absence of high-caloric/-fat diet. In total, the systematic review comprises 21 articles (n = ~354, one article did not state “n” [33], none of them considered as gray literature) and 19 were eligible for meta-analysis (n = 338). Ten trials were conducted in a parallel group design whereas the remaining eleven applied randomization. Rodent strains involved Sprague Dawley rats (n = 5), Wistar rats (n = 3), C57BL/6 J mice (n = 10), ddY mice (n = 1), ICR mice (n = 1) and BALB/c mice (n = 1). Rodent age ranged from 5 to 13 (average of 7.7) weeks after acclimatization at the start of the experimental intervention. Supplementation phase lasted 3 to 20 weeks with an average of 2 months. Nineteen studies provided high-fat or HDCs. One study used a normal control diet [34] and one trial did not state the type of diet [35], but due to appropriate study designs, we decided to include them. The majority of experiments produced and/or administered marine-derived CPs, the minority being extracted from porcine, sheep, chicken, bovine and yak sources.
Body mass and subgroup analysis
Eighteen studies (n = 318) investigated CPs influence on rodent’s body mass. As seen in Fig. 1A and Table 1, body mass has been reduced significantly in the CP group (p < 0.01; SMD = −1.87; CI = −2.8, −0.94). High heterogeneity was observed (I² = 84.9%) as well as potential publication bias (p < 0.01, Fig. S2). Subgroup analysis (Table 2) showed no significant changes when correcting for species, intervention duration and age. CP source influenced heterogeneity when studies administering fish-derived CP (and studies which did not provide data on the source) were excluded, reaching an I² of 53.9% (moderate). Simultaneously, SMD changed to −0.6 and p = 0.07 with a CI of −1.25, 0.06. Therefore, supplement “type” as a particular subgroup reached significance of p = 0.02, implying supplement “type” to be a potential source of heterogeneity.
Glucose metabolism
Eight studies involving 120 rodents revealed a significantly alleviated glucose metabolism in the CP group (Fig. 1B; p = 0.02; SMD = −2.21; CI = −3.92, −0.49), although potential publication bias (p < 0.01, Fig. S3) and heterogeneity (I² = 91.6%) were considered high.
Food intake
Ten trials comprising 192 rodents elicited a significantly reduced food intake in the CP supplementation group (Fig. 1C; p = 0.01; SMD = −1.43; CI = −2.56, −0.3), even though heterogeneity was found to be high (I² = 88%) and publication bias existed (p = 0.02, Fig. S2).
Adipose tissue and organ content and adipocyte size
Ten trials including 176 rodents demonstrated a significantly lower amount of adipose tissue and organ content when administering CPs (Fig. 2A; p < 0.01; SMD = −1.26; CI = −1.78, −0.75). Publication bias was evident (p < 0.01, Fig. S3). Heterogeneity was also given to a high extent (I² = 75.2%). Five studies examining adipocyte size in 112 rodents led to a non-significant result between both control and CP group (Fig. 2B; p = 0.053; SMD = −2.21; CI = −4.44, 0.03). High heterogeneity (I² = 91.4%) and possibly no potential publication bias (p = 0.3, Fig. S2) was detected.
Serum lipids
Twelve studies comprising 188 rodents investigating total cholesterol reported no significant differences between control and CP group (Fig. 3A; p = 0.08; SMD = −1.49; CI = −3.17, 0.18). Heterogeneity remained high (I² = 90.8%) and Egger’s regression test did not hint to publication bias (p = 0.39, Fig. S2). Seven trials measuring LDL levels in 118 rodents resulted in a significantly lower LDL amount following prolonged CP administration (Fig. 3B; p < 0.01; SMD = −2.02; CI = −3.15, −0.88). However, high heterogeneity (I² = 80.2%) and publication bias were calculated (p = 0.01, Fig. S2). High-density lipoprotein (HDL) gauged in 98 rodents in six studies has been shown to be elevated in CP groups (Fig. 3C, p = 0.04; SMD = 1.14; CI = 0.06, 2.21). Nonetheless, high heterogeneity (I² = 81.8%) and publication bias were present (p = 0.04, Fig. S2). Regarding triacylglycerol (TG), 13 studies involving 204 rodents demonstrated a significantly lower TG in the CP group (Fig. 4A; p < 0.01; SMD = −2.16; CI = −3.76, −0.55). Publication bias (p < 0.01, Fig. S2) and high heterogeneity (I² = 87.9%) existed.
Adipokines (leptin and adiponectin)
Four studies (64 rodents) investigating adipokines (leptin and adiponectin) showed significant differences following CP supplementation for adiponectin (increased, p < 0.01; SMD = 1.04; CI = 0.47, 1.6) and leptin (decreased, p = 0.01; SMD = −1.33; CI = −2.36, −0.31; both Fig. 4B, C, respectively). Very low (I² = 22.3%) and moderate (I² = 65.6%) heterogeneity was calculated for adiponectin and leptin, respectively. Publication bias was not observed in both adiponectin (p = 0.6) and leptin (p = 0.21, both Fig. S2).
Oxidative capacity
A non-significantly higher oxidative capacity could be disclosed in the CP group of seven studies, including 120 rodents (Fig. 5A; p = 0.16; SMD = 1.83; CI = −0.77, 4.42). A high heterogeneity (I² = 98.5%) and no publication bias (p = 0.09, Fig. S3) were observed.
A Oxidative capacity (signs of TBARS, ROS and MDA have been reversed since lower levels exhibit better oxidative protection). B Inflammation (signs of IL-4 and IL-10 have been reversed since higher levels exhibit an enhanced anti-inflammatory state). C Lipid metabolism related transcription factors (sign of PPARα has been reversed since higher levels exhibit enhanced lipid metabolism).
Inflammation
Five studies examining the inflammatory status of 80 rodents illustrated no significantly anti-inflammatory effect of CP administration compared to HFD/ HFC only (Fig. 5B; p = 0.22; SMD = −3.82; CI = −10.27, 2.63). Publication bias (p < 0.01, Fig. S3) and high heterogeneity (I² = 99.1%) were apparent.
Lipid metabolism-related transcription factors
The pooled expression of specific transcription factor involved in lipid metabolism and energy balancing in four studies comprising 64 rodents were not found to be significantly downregulated when CP was supplemented (Fig. 5C; p = 0.15; SMD = 2.36; CI = −5.72, 0.99). Heterogeneity was high (I² = 98%) and publication bias evident (p < 0.01, Fig. S3).
Microbiome
The Firmicutes/Bacteroidetes ratio as a descriptor of the gut microbiome was measured in three studies, with one not stating the number of rodents [33]. The results (PLA = 6.75 CP = 1.64 [26], PLA = 10.41 ± 4.01 CP = 5.26 ± 1.33 [33], PLA = 0.57 CP = 1.5 [36]) suggest no clear direction since two studies found higher ratios (both significant) for control groups and one study for the CP group (not significant). A dose-dependent manner was evident for a decreasing F/B ratio with increasing CP dose [26].
Risk of bias and certainty of evidence
The risk of bias, which was assessed by using the SYRCLE tool [28] that has been developed (and adapted based on Cochranes RoB tool) especially for preclinical animal experiments, can be considered rather high (Table 3). Items 3–7 have been marked as “unclear” in every single study (except Wang et al.13, since only the abstract, which included all the data, was available). The corresponding authors did not provide any information (after written request) in the full-text manuscripts regarding allocation concealment, random housing, (investigator/caregiver) blinding, random outcome assessment and blinding of outcome assessor. Most of the trials (n = 20) were found to be free of selective outcome reporting and showed groups being similar baseline characteristics (n = 17). A method to generate an allocation sequence was only applied and mentioned in half of the trials. With respect to other sources of bias, two studies declared to have been funded by the CP manufacturer. Otherwise, no other sources have been recognized. The certainty of evidence evaluated by means of the GRADE approach appears to be very low overall (Table 1). This is due to high risk of bias, inconsistency and indirectness (due to analysis of animals only) of results, imprecision and possible publication bias (at least for analyses including more than 10 studies).
Discussion
To our knowledge, this is the first systematic review and meta-analysis to investigate the effects of long-term CP administration in rodents on a high-fat or HCD. The findings suggest that CP supplementation appears to significantly decrease body mass, food intake, leptin, adipose tissue content, LDL and TG. Enhanced adiponectin levels and attenuated glucose metabolism have been observed. However, the overall certainty of evidence seemed very low.
Effects on body mass, food intake, leptin and adipose tissue
The results of the current meta-analysis show CPs superiority in decreasing rodent body weight, food intake and adipose tissue. A several-week administration of sole glycine, the most abundant amino acid in CPs, even showed a pronounced loss of whole-body fat and epididymal fat while preserving lean mass and quadriceps muscle mass in mice facing obesity undergoing caloric restriction [37]. Glycine intake in mice has also been reported to prevent maturity-onset obesity and hepatic steatosis [38], a disease elicited by fatty acid and TG accumulation within the liver that might ultimately result in cirrhosis and even hepatocellular carcinoma [39]. Glycine also facilitates excitatory transmission in the brain via activation of the N-methyl-d-aspartate receptor [40], which seems to be involved in the regulation of food intake [41]. In addition, glycine receptor (GlyR) protein levels were augmented in the hypothalamus of HFD-fed mice, implicating a potential role of GlyR in obesity-related orexogenic signaling [42]. Therefore, glycine as a primary source of CP could also be of interest for human clinical testing in the context of obesity [43].
Furthermore, an in-vitro trial delivered evidence of hydrolyzed collagen (HC) conjugated with epigallocatechin gallate inhibiting the activity of obesity associated enzymes α-amylase and α-glucosidase [44], but with reduced inhibitory potential when HC was applied alone. In human exercise trials lasting several months where subjects typically exercise three times a week, daily CP administration has significantly augmented lean body mass [45], evidenced by numerous experiments, even while reducing fat mass (FM) [46, 47]. Therefore, CP supplementation could be a beneficial adjunct for patients with obesity as well, and in fact, a recent study has demonstrated a significantly reduced FM following a 12-week CP regimen compared to a placebo in middle-aged, untrained men with obesity [48]. Body weight reductions alongside decreased body FM [49] have been reported following 12-weeks of daily CP intake in adults ranging from normal weight to overweight [50] without concomitant significant declines in food intake [51] expressed as total kcal consumed. In another human randomized controlled trial, individuals with obesity who did not receive a prescribed exercise intervention were administered bovine collagen—technologically modified to enhance its water-binding capacity—over a period of 12 weeks. Although energy and macronutrient intake did not differ significantly between groups after the intervention, the collagen group experienced significantly greater weight loss (~1.5 kg), increased satiety and reduced hunger (both assessed by questionnaire data). Additionally, in a preceding experiment, postprandial blood ghrelin levels of rats were also significantly lower following collagen intake compared to casein [52]. Together with the aforementioned findings, this highlights collagen protein’s potential to promote satiety through its distinct physical properties in humans. However, it remains to be elucidated whether effects on orexigenic hormones also occur in humans.
Leptin, a peptide hormone mainly produced in adipose tissue and involved in the regulation of body mass and food intake, was significantly reduced by CP intake in the present meta-analysis. This is in line with trials including subjects with obesity [53] and massive obesity [54] (BMI of 35 and above), illustrating higher serum leptin and mRNA expression levels (ex vivo) than normal-weight controls and a strong positive association between blood leptin concentrations and body fat percentage. The mRNA content of the ob gene (responsible for leptin production) was also twice as high in the group with obesity, suggesting subjects with obesity have developed leptin resistance that might be the consequence of lowered leptin receptor expression or disturbed leptin receptor signaling [55]. Since mechanisms regarding leptin and CPs are currently unknown, we suppose that CP-induced reductions in weight and food intake might have caused concomitant low leptin levels, at least in rodents. Moreover, the significant decrease in adipose tissue and organ content in the current meta-analysis may have contributed to low leptin levels since adipose tissue is majorly involved in leptin production. Furthermore, adipose tissue organ content and adipocyte diameter or cross-sectional area, usually gauged ex vivo, are often not investigated in human trials, which dampens the transferability of potential CP effects in humans. Apart from that, mixed results have been reported from two long-term human trials supplementing CPs for 10–12 weeks in individuals with a BMI above 25. In one study, leptin levels decreased in both groups but remained significantly higher in the CP group compared to placebo [52]; in the other, leptin levels remained stable, with a tendency to increase in the whey protein group compared to CP [56]. Hence, robust human clinical evidence is still lacking regarding the effects of CPs on leptin regulation and related metabolic outcomes.
Effects on serum lipids and glucose metabolism
The current meta-analysis revealed significant reductions in serum lipid markers (LDL and TG). Additionally, HDL levels increased, while total cholesterol levels remained largely unchanged under the caveat of high heterogeneity. One underlying mechanism appears to be CPs potential influence on lipid absorption, which may be effective in suppressing transient blood TG increases as demonstrated in rats [57]. Plasma TG concentration has been reported to negatively correlate (r = 0.53–0.63) with plasma glycine, proline and hydroxyproline, highlighting fish-derived CP amino acids’ ability to counteract TG accumulation [57]. Fish CPs, especially Hyp- comprising peptides, have also been found in higher abundance in human blood compared to porcine-derived CPs [58]. As higher levels of blood CPs might inhibit TG absorption, low-molecular-weight (to enhance absorption) fish-specific CP administration could provide lipid-lowering effects in obesity. As certain lipid markers have also been improved in the current review of preclinical studies, such findings have yet to be observed in human trials [49,50,51], particularly those involving subjects with obesity [56], since dyslipidemia displays a crucial risk factor for developing cardiovascular diseases and is even associated with non-alcoholic fatty liver disease and acute pancreatitis [59].
Alleviated glucose metabolism (expressed as glucose, insulin and glycated hemoglobin (HbA1c)) was also observed in the present meta-analysis. High-dose CPs (1.7 g/kg bodyweight) derived from tilapia skin exhibited similar glucose-lowering effects evoked by metformin in diabetic mice after 25 days [60]. CPs from harpadon nehereus bones elicited favorable up- and downregulations of certain key enzymes following a 4-week administration in diabetes type 1 (DMT1) mouse models. The CP-induced expression levels of glucose-6-phosphatase (G6Pase) and phosphoenolpyruvate carboxykinase 1 (PEPCK1) in the liver of the diabetic mice were reduced, while the Glucokinase (GK) and phosphorylation of Glycogen synthase kinase-3 (GSK-3β) were elevated. In addition, apoptosis in pancreatic cells and hepatocytes as well as cell swelling and alanine transaminase (ALT) & aspartate aminotransferase (AST) could be lowered, which might indicate CPs to ameliorate DMT1-induced liver damage [61]. In a human trial, 5 g of fish CPs were also able to improve resting blood glucose and insulin sensitivity in adults with DMT2 following a 90-day supplementation phase [62]. These results, plus reduced HbA1c levels, were also obtained in Chinese adults with DMT2 and normal weight after 6 and 9 months of daily 13 g marine CP supplementation [63]. At least in patients with diabetes, CPs from sheep skin have been shown to inhibit dipeptidyl peptidase-IV (DPP-IV) activity [64] that prolongs and increases the activity of the incretin hormones glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide, which act as key prandial stimulators of insulin secretion and regulators of blood glucose control [65]. Fish-derived CPs might thus be a suitable supplement for individuals with DMT2 [66] and probably for patients with obesity and dysregulated glucose metabolism. Nevertheless, glucose metabolism has not been influenced by an 8-week CP administration regimen in adults with overweight, demonstrated by unchanged levels of glucose, insulin and the homeostatic model assessment of insulin resistance (HOMA-IR) and β cell function (HOMA-β; both being markers of insulin dynamics [56, 67]).
Effects on inflammation, oxidative stress, and adiponectin
Somewhat surprisingly, significant anti-inflammatory effects as well as enhanced oxidative capacity following CP administration have not been observed in the present meta-analysis. In a mouse model treated with D-galactose together with UV irradiation, a 7-week chicken bone-derived CP administration inhibited skin inflammation and improved levels of antioxidants. Moreover, lysosome-related genes were upregulated, indicating enhanced degradation of cellular debris/protein aggregates [68]. Other than the investigated inflammatory and oxidative parameters in the current review, an in-vitro experiment referred to CPs (extracted from chicken sternal cartilage) ability to scavenge ABTS and DPPH radical (two common assays used for measuring radical scavenging) and alleviating H2O2-induced cellular oxidative damage in rat knee joints, probably caused by low-molecular-weight and hydrophobic & antioxidant amino acid residues. Pro-inflammatory cytokine secretion was also decreased, probably elicited from increased synthesis of extracellular matrix key components combined with suppressed chondrocyte apoptosis [69]. Milkfish CPs also revealed good ABTS and DPPH radical scavenging as well as reductions in lipoxygenase activity, nitric oxide radicals, and DNA single-strand breaks in vitro [23]. A recent advance by using ultrasound-assisted (450 W) enzymatic hydrolysis for CP preparation demonstrated enhanced peptide and α-helix content together with decreased random coil and β-chain, thus likely acting anti-inflammatory in lipopolysaccharide (LPS)-induced cells [70]. LPS- induced inflammation was also mitigated in mice by Tilapia-derived CPs [71]. Inflammation specifically taking place in the colon of ulcerative colitis-positive mice has been ameliorated by CP-provoked upregulation of mitogen-activated protein kinase phosphatase-1 (MKP-1) and by that reducing the phosphorylation levels of both signaling proteins c-Jun N-terminal kinases (JNK) and p38 mitogen-activated protein kinases (P38), finally attenuating pro-inflammatory cytokine expression (TNF-α, IL-1β, and IL-6) [72]. A recent investigation in Dextran Sodium Sulfate induced colitis mice reported fish CPs acting protectively against colitis by directly influencing macrophages, steering their polarization towards an anti-inflammatory, immunotolerant, and antioxidant phenotype in a mannose receptor-dependent manner. Additionally, CPs impact on the immune system may help sustain intestinal eubiosis [73]. Another approach in attenuating inflammation seems to be targeting the nuclear factor kappa-B (NF-κB) signaling pathway. In acute kidney injury mice [74] and human HaCaT keratinocyte cells [75], CPs diminished NF-κB along with other pro-inflammatory cytokines. Furthermore, six peptides (GPAGPSGPAGK, GPAGPSGPAGKDGR, GPSGPQGIR, GPAGPQGPR, GEAGPAGPAGPAGPR, and GEGGPQGPR) have been shown to regulate NF-κB signaling pathways together with nitric oxide production to act anti-inflammatorily [76]. Nonetheless, human trials on CP supplementation have not yet reported significant anti-inflammatory effects, at least following muscle damage-induced inflammation [77, 78]. Two 24-week multicenter trials in patients with rheumatoid arthritis—a chronic autoimmune and inflammatory joint disease [79]—reported improved joint function and enhanced therapeutic efficacy following supplementation with CP (derived from type II collagen). These effects were observed even at low doses of 20 µg [80] and 100 µg/d [81]. Although CRP levels did not decrease in one of the trials, the results provide preliminary indirect evidence for the anti-inflammatory potential of CPs in humans.
Adiponectin, a hormone synthesized in white adipose tissue, has been found significantly elevated after CP administration. Glycine, as the major amino acid in collagen, has been reported to significantly enhance adipocyte-released adiponectin mRNA in a 3T3-L1 cell line [82]. Adiponectin has already been reported to improve insulin sensitivity, enhance fatty acid transportation, provide anti-atherosclerosis effects and even modulate inflammatory responses by means of targeting mast cells, eosinophils and macrophages [83, 84]. Its anti-inflammatory properties might derive from its ability to lower CRP and its corresponding mRNA, inhibiting NF-κB signaling and macrophage-specific secretion of TNFα [85]. Ostensibly, mainly glycine from CPs might foster these processes as this collagen-specific amino acid has also demonstrated enhanced mRNA levels of adiponectin and IL-10 without affecting adipogenesis in vitro [86], suppression of pro-inflammatory cytokines production and increasing adiponectin secretion in vivo via activation of PPARγ in mice [87]. In a human cross-sectional study [88], a negative correlation has been found between adiponectin and fasting plasma glucose in metabolically healthy individuals with obesity (those without metabolic syndrome). However, a recent review highlighted paradoxical effects of both adipokines, suggesting that high or low levels may not actually confer beneficial effects on cardiovascular function [89], which is why therapeutic agents might seek a slight adaptation of these cytokines. To our knowledge, just a single study has investigated CPs impact on adipokines in women with overweight following an 8-week administration regimen, resulting in unchanged leptin and adiponectin levels (accompanied with stabilized lean body mass) [56]. CPs mechanistic influence on adiponectin and vice versa and their role in obesity is still not fully understood.
Effects on transcription factors involved in lipid metabolism
Lipid metabolism-related transcription factors have not revealed a significant decrease in the present systematic review. The peroxisome proliferator-activated receptor (PPAR) family are widely known as metabolic regulators, modulating lipid homeostasis. PPARα & γ, which were included in the meta-analysis, are mainly responsible for fatty acid β-oxidation and the storage of TGs in adipocytes, respectively [90]. PPARγ, together with CCAAT/enhancer binding protein α (C/EBPα) mRNA and protein expression, another key transcription factor in adipogenesis [91], could be downregulated in the studies included in the current analysis. PPARα upregulation was also achieved with CPs in the liver of db/db mice alongside a downregulation of SREBP-1 & 2, indicating a substantial suppression of hepatic lipid accumulation [92]. An animal investigation of glycine administration alone resulted in a reduction of PPARγ mRNA expression in the liver after 4 and 8 weeks, but not in adipose tissue of monosodium glutamate-induced mice with obesity (MSG/Ob mice) compared to lean ones [93]. Surprisingly, liver PPARα mRNA expression significantly decreased in these MSG/Ob mice, indicating that the anti-obesity effects of CPs may be attributed to amino acids or peptides other than glycine. Prolyl-hydroxyproline (Pro-Hyp), a bioactive peptide abundantly found in CPs, has recently been reported to decrease adipocyte size, to increase mitochondrial activity and to upregulate brown fat-specific genes such as C/EBPα and PPARγ coactivator-1 alpha (PGC-1α) without altering PPARγ expression. Surprisingly, a Pro-Hyp-responsive element was identified in the PGC-1α gene promoter, enabling Foxg1 (= transcription factor) to bind and increase PGC-1α expression. This, in turn, promotes brown adipocyte differentiation, highlighting a potential anti-obesity effect—at least in an in vitro setting [94]. Currently, it remains unknown whether CPs or collagen-specific amino acids exert any influence on lipid metabolism-related transcription factors in humans.
Effects on the gut microbiome
Microbiome analysis revealed equivocal findings in this systematic review, exhibiting lower Firmicutes/Bacteroidetes ratios after CP intake in two out of three studies. A high-fat diet commonly leads to a Firmicutes (Clostridium) dominant gut microbiota, while being deficient in beneficial genera/species such as Bacteroides, Bifidobacterium or Lactobacillus. This altered profile of the gut is associated with decreased short-chain fatty acids, which are essential for maintaining intestinal epithelial barrier integrity, reducing inflammation and bacterial translocation, and increasing hunger-suppressing hormone expression [95]. In an iron-deficiency anemia rat model, inducing high abundances of Firmicutes, a chelate of pig skin CP and Fe2+ supplemented for 3 weeks, conveyed a higher number of Bacteroidetes and fixed the iron-deficiency anemia evoked decrease in the relative abundances of Firmicutes and Bacteroidetes [96]. A downgrade of Subdoligranulum relative abundance, a bacteria that might be relevant for mitigating DMT2 and obesity [97], was also observed with CP intake in this experiment. To the best of our knowledge, only a single trial has examined the impact of a 2-week CP intervention on the gut microbiota, specifically in patients suffering from major burn injuries [98]. Bifidobacterium levels significantly decreased in both the placebo and collagen + sunflower oil groups, whereas no change was observed in the collagen + fish oil group. Notably, the Firmicutes/Bacteroidetes ratio remained stable only in the collagen + fish oil group, likely due to its high omega-3 content. However, further human trials investigating potential microbiota changes following prolonged CP supplementation are still lacking, leaving the effects on the human microbiome and obesity largely uncertain.
Limitations
As preclinical animal studies differ in several aspects (e.g., species, design, age, etc. [99]) from clinical human trials, mostly high heterogeneity was observed throughout the current meta-analysis. The subgroup analysis undertaken for one parameter having the highest number of studies included revealed that disparities in intervention length and CP source existed, which might have caused high between-study variability and total variation. An overall poor methodological quality was present, which substantially enlarged the risk of bias. Moreover, many authors have not responded to our written requests, which is why a lot of data had to be extracted with the web plot digitizer tool, a procedure that might have also elicited some variability. The certainty of evidence assessed by the GRADE approach remained very low for each parameter due to inconsistency, indirectness and risk of bias. Recent research on animal-to-human translation reported a median success rate of approximately 65%, with a range from 0 to 100% [100]. In light of the aforementioned limitations, translating the present findings to humans remains particularly challenging [101]. However, the doses applied in the analyzed trials ranged from 0.01 to 4.5 g/kg body weight in rodents, corresponding to a maximum human equivalent dose of approximately 51 g for a 70 kg individual [102]—a dose that has already been administered in human clinical trials [103, 104]. Lastly, the CPs included in the present review were extracted from various animal sources, each potentially exerting distinct effects [105]. The identification of novel di-, tri-, and oligopeptides with efficacy against metabolic disorders in humans remains an important subject for future research.
Conclusion
CP administration in rodent obesity models of at least 3 weeks demonstrated significant beneficial effects with respect to body mass, food intake, adipose tissue content, endocrine functioning, adipokines, glucose metabolism, and lipid serum markers. More research is definitely needed in the field of CP-related anti-obesity effects in human trials, given that a handful of studies majorly examined blood lipids and glucose together with body composition (body weight, fat & fat-free mass) variables. Although not significantly enhanced in the current meta-analysis (which included only four studies), CPs may influence lipid metabolism through the molecular regulation of specific transcription factors. Future studies are encouraged to further investigate the molecular mechanisms of CP supplementation in humans. Future preclinical trials should highly consider randomization of animals and blinding of researchers and following the ARRIVE guidelines 2.0 [106] to improve overall reporting quality, since reporting prevalence is still lacking in experimental animal research [107].
Data availability
The datasets generated during the current systematic review and meta-analysis are available from the corresponding author on reasonable request.
References
Lobstein T, Jackson-Leach R, Powis J, Brinsden H, Gray M. World Obesity Atlas 2023. Available from: https://data.worldobesity.org/publications/?cat=19.
Okunogbe A, Nugent R, Spencer G, Powis J, Ralston J, Wilding J. Economic impacts of overweight and obesity: current and future estimates for 161 countries. BMJ Glob Health. 2022;7:e009773.
Lin X, Li H. Obesity: epidemiology, pathophysiology, and therapeutics. Front Endocrinol. 2021;12:706978.
Speakman JR, Sørensen TIA, Hall KD, Allison DB. Unanswered questions about the causes of obesity. Science. 2023;381:944–6.
Roberge J-B, van Hulst A, Barnett TA, Drapeau V, Benedetti A, Tremblay A, et al. Lifestyle habits, dietary factors, and the metabolically unhealthy obese phenotype in youth. J Pediatr. 2019;204:46–52.e1.
Simonson M, Boirie Y, Guillet C. Protein, amino acids and obesity treatment. Rev Endocr Metab Disord. 2020;21:341–53.
Wong RPM, Zhou ZK, Strappe PM. The anti-obesogenic and anti-diabetic properties of marine collagen peptides. Front Food Sci. Technol. 2024;3:1270392.
Vijayan DK, Perumcherry Raman S, Dara PK, Jacob RM, Mathew S, Rangasamy A, et al. In vivo anti-lipidemic and antioxidant potential of collagen peptides obtained from great hammerhead shark skin waste. J Food Sci Technol. 2022;59:1140–51.
Axarlis K, Daskalaki MG, Michailidou S, Androulaki N, Tsoureki A, Mouchtaropoulou E, et al. Diet supplementation with fish-derived extracts suppresses diabetes and modulates intestinal microbiome in a murine model of diet-induced obesity. Mar Drugs. 2021;19:268.
Sladoje DP, Kisić B, Mirić D. The monitoring of protein markers of inflammation and serum lipid concentration in obese subjects with metabolic syndrome. J Med Biochem. 2017;36:366–74.
Glew RH, Kassam HA, Bhanji RA, Okorodudu A, VanderJagt DJ. Serum lipid profiles and risk of cardiovascular disease in three different male populations in northern Nigeria. J Health Popul Nutr. 2002;20:166–74.
Lee EJ, Hur J, Ham SA, Jo Y, Lee S, Choi M-J, et al. Fish collagen peptide inhibits the adipogenic differentiation of preadipocytes and ameliorates obesity in high-fat diet-fed mice. Int J Biol Macromol. 2017;104:281–6.
Wang J, Xie Y, Pei X, Yang R, Zhang Z, Li Y. The lipid-lowering and antioxidative effects of marine collagen peptides. Zhonghua Yu Fang Yi Xue Za Zhi. 2008;42:226–30.
Lustig RH, Collier D, Kassotis C, Roepke TA, Kim MJ, Blanc E, et al. Obesity i: overview and molecular and biochemical mechanisms. Biochem Pharm. 2022;199:115012.
Woo M, Song YO, Kang K-H, Noh JS. Anti-obesity effects of collagen peptide derived from skate (Raja kenojei) skin through regulation of lipid metabolism. Mar Drugs. 2018;16:306.
Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31–55.
Suryaningtyas IT, Je J-Y. Bioactive peptides from food proteins as potential anti-obesity agents: mechanisms of action and future perspectives. Trends Food Sci Technol. 2023;138:141–52.
Zhu C, Zhang W, Mu B, Zhang F, Lai N, Zhou J, et al. Effects of marine collagen peptides on glucose metabolism and insulin resistance in type 2 diabetic rats. J Food Sci Technol. 2017;54:2260–9.
Liu H, Yang Y, Liu Y, Cui L, Fu L, Li B. Various bioactive peptides in collagen hydrolysate from Salmo salar skin and the combined inhibitory effects on atherosclerosis in vitro and in vivo. Food Res Int. 2022;157:111281.
Hussain T, Tan B, Yin Y, Blachier F, Tossou MCB, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us?. Oxid Med Cell Longev. 2016;2016:7432797.
Seop JeongKap, Sook NohJeong. Anti-inflammatory effect of skate collagen peptide through attenuation of oxidative stress. 한국유화학회지. 2018;35:1369–78.
Wang B, Wang Y-M, Chi C-F, Luo H-Y, Deng S-G, Ma J-Y. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar Drugs. 2013;11:4641–61.
Chen Y-P, Liang C-H, Wu H-T, Pang H-Y, Chen C, Wang G-H, et al. Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J Food Sci Technol. 2018;55:2310–7.
Murphy EA, Velazquez KT, Herbert KM. Influence of high-fat diet on gut microbiota: a driving force for chronic disease risk. Curr Opin Clin Nutr Metab Care. 2015;18:515–20.
Mei F, Duan Z, Chen M, Lu J, Zhao M, Li L, et al. Effect of a high-collagen peptide diet on the gut microbiota and short-chain fatty acid metabolism. J Funct Foods. 2020;75:104278.
Guo Z, Hu B, Zhu L, Yang Y, Liu C, Liu F, et al. Microbiome-metabolomics insights into the feces of high-fat diet mice to reveal the anti-obesity effects of yak (Bos grunniens) bone collagen hydrolysates. Food Res Int. 2022;156:111024.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023) 2023. https://training.cochrane.org/handbook.
Hooijmans CR, Rovers MM, Vries RBM, de, Leenaars M, Ritskes-Hoitinga M, et al. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43.
Granholm A, Alhazzani W, Møller MH. Use of the GRADE approach in systematic reviews and guidelines. Br J Anaesth. 2019;123:554–9.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Quintana DS. From pre-registration to publication: a non-technical primer for conducting a meta-analysis to synthesize correlational data. Front Psychol. 2015;6:1549.
Sudakov SK, Alekseeva EV, Nazarova GA, Bashkatova VG. Age-related individual behavioural characteristics of adult Wistar rats. Animals. 2021;11:2282.
Baek GH, Yoo KM, Kim S-Y, Da Lee H, Chung H, Jung S-C et al. Collagen peptide exerts an anti-obesity effect by influencing the firmicutes/bacteroidetes ratio in the gut. Nutrients. 2023;15:2610.
Tometsuka C, Funato N, Mizuno K, Taga Y. Long-term intake of ginger protease-degraded collagen hydrolysate reduces blood lipid levels and adipocyte size in mice. Curr Res Food Sci. 2021;4:175–81.
Chiang T-I, Chang I-C, Lee H-H, Hsieh KH, Chiu Y-W, Lai T-J, et al. Amelioration of estrogen deficiency-induced obesity by collagen hydrolysate. Int J Med Sci. 2016;13:853–7.
Wang S, Lv Z, Zhao W, Wang L, He N. Collagen peptide from Walleye pollock skin attenuated obesity and modulated gut microbiota in high-fat diet-fed mice. J Funct Foods. 2020;74:104194.
Caldow MK, Ham DJ, Godeassi DP, Chee A, Lynch GS, Koopman R. Glycine supplementation during calorie restriction accelerates fat loss and protects against further muscle loss in obese mice. Clin Nutr. 2016;35:1118–26.
Takashima S, Ikejima K, Arai K, Yokokawa J, Kon K, Yamashina S, et al. Glycine prevents metabolic steatohepatitis in diabetic KK-Ay mice through modulation of hepatic innate immunity. Am J Physiol Gastrointest Liver Physiol. 2016;311:G1105–G1113.
Zhou X, Han D, Xu R, Wu H, Qu C, Wang F, et al. Glycine protects against high sucrose and high fat-induced non-alcoholic steatohepatitis in rats. Oncotarget. 2016;7:80223–37.
Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–31.
Sorrels TL, Bostock E. Induction of feeding by 7-chlorokynurenic acid, a strychnine-insensitive glycine binding site antagonist. Brain Res. 1992;572:265–8.
Manousopoulou A, Koutmani Y, Karaliota S, Woelk CH, Manolakos ES, Karalis K, et al. Hypothalamus proteomics from mouse models with obesity and anorexia reveals therapeutic targets of appetite regulation. Nutr Diab. 2016;6:e204.
Alves A, Bassot A, Bulteau A-L, Pirola L, Morio B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients. 2019;11:1356.
Chotphruethipong L, Binlateh T, Hutamekalin P, Sukketsiri W, Aluko RE, Benjakul S. Hydrolyzed collagen from defatted sea bass skin and its conjugate with epigallocatechin gallate: in vitro antioxidant, anti-inflammatory, wound-healing and anti-obesity activities. Food Biosci. 2021;43:101303.
Jendricke P, Kohl J, Centner C, Gollhofer A, König D. Influence of specific collagen peptides and concurrent training on cardiometabolic parameters and performance indices in women: a randomized controlled trial. Front Nutr. 2020;7:580918.
Zdzieblik D, Oesser S, Baumstark MW, Gollhofer A, König D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: a randomised controlled trial. Br J Nutr. 2015;114:1237–45.
Jendricke P, Centner C, Zdzieblik D, Gollhofer A, König D. Specific collagen peptides in combination with resistance training improve body composition and regional muscle strength in premenopausal women: a randomized controlled trial. Nutrients. 2019;11:892.
Zdzieblik D, Jendricke P, Oesser S, Gollhofer A, König D. The influence of specific bioactive collagen peptides on body composition and muscle strength in middle-aged, untrained men: a randomized controlled trial. Int J Environ Res Public Health. 2021;18:4837.
Tak YJ, Kim YJ, Lee JG, Yi Y-H, Cho YH, Kang GH et al. Effect of oral ingestion of low-molecular collagen peptides derived from skate (Raja Kenojei) skin on body fat in overweight adults: a randomized, double-blind, placebo-controlled trial. Mar Drugs 2019;17:157.
Park J, Kim M, Shin H, Ahn H, Park YK. Low-molecular collagen peptide supplementation and body fat mass in adults aged ≥50 years: a randomized, double-blind, placebo-controlled trial. Clin Nutr Res. 2023;12:245–56.
Han CJ, Kang SM. The effect of collagen supplementation from pork skin on serum collagen, serum sex steroid hormone, serum lipid and skin crack in Korean middle-aged women. Korean J Community Nutr. 2008;13:912–21.
López-Yoldi M, Riezu-Boj JI, Abete I, Ibero-Baraibar I, Aranaz P, González-Salazar I et al. Anti-obesity effects of a collagen with low digestibility and high swelling capacity: a human randomized control trial. Nutrients. 2024;16:3550.
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5.
Hamilton BS, Paglia D, Kwan AY, Deitel M. Increased obese mRNA expression in omental fat cells from massively obese humans. Nat Med. 1995;1:953–6.
Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, et al. Leptin and obesity: role and clinical implication. Front Endocrinol. 2021;12:585887.
Giglio BM, Schincaglia RM, da Silva AS, Fazani ICS, Monteiro PA, Mota JF et al. Whey protein supplementation compared to collagen increases blood nesfatin concentrations and decreases android fat in overweight women: a randomized double-blind study. Nutrients. 2019;11:2051.
Saito M, Kiyose C, Higuchi T, Uchida N, Suzuki H. Effect of collagen hydrolysates from salmon and trout skins on the lipid profile in rats. J Agric Food Chem. 2009;57:10477–82.
Ohara H, Matsumoto H, Ito K, Iwai K, Sato K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J Agric Food Chem. 2007;55:1532–5.
Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021;18:689–700.
Zhang R, Chen J, Jiang X, Yin L, Zhang X. Antioxidant and hypoglycaemic effects of tilapia skin collagen peptide in mice. Int J Food Sci Tech. 2016;51:2157–63.
Lin Q, Guo Y, Li J, He S, Chen Y, Jin H. Antidiabetic effect of collagen peptides from harpadon nehereus bones in streptozotocin-induced diabetes mice by regulating oxidative stress and glucose metabolism. Mar Drugs 2023;21:518.
Devasia S, Kumar S, Stephena PS, Inoue N, Sugihara F, Koizumi S, et al. A double blind, randomised, four arm clinical study to evaluate the safety, efficacy and tolerability of collagen peptide as a nutraceutical therapy in the management of type II diabetes mellitus. J Diab Metab. 2020;10:839.
Zhu C-F, Li G-Z, Peng H-B, Zhang F, Chen Y, Li Y. Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl Physiol Nutr Metab. 2010;35:797–804.
Wang B, Yu Z, Yokoyama W, Chiou B-S, Chen M, Liu F, et al. Collagen peptides with DPP-IV inhibitory activity from sheep skin and their stability to in vitro gastrointestinal digestion. Food Biosci. 2021;42:101161.
Green BD, Flatt PR, Bailey CJ. Dipeptidyl peptidase IV (DPP IV) inhibitors: a newly emerging drug class for the treatment of type 2 diabetes. Diab Vasc Dis Res. 2006;3:159–65.
Xu S, Zhao Y, Song W, Zhang C, Wang Q, Li R, et al. Improving the sustainability of processing by-products: extraction and recent biological activities of collagen peptides. Foods. 2023;12:1965.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9.
Cao C, Xiao Z, Tong H, Liu Y, Wu Y, Ge C. Oral intake of chicken bone collagen peptides anti-skin aging in mice by regulating collagen degradation and synthesis, inhibiting inflammation and activating lysosomes. Nutrients. 2022;14:1622.
Wang J, Luo D, Liang M, Zhang T, Yin X, Zhang Y, et al. Spectrum-effect relationships between high-performance liquid chromatography (HPLC) fingerprints and the antioxidant and anti-inflammatory activities of collagen peptides. Molecules. 2018;23:3257.
Hao Y, Xing L, Wang Z, Cai J, Toldrá F, Zhang W. Study on the anti-inflammatory activity of the porcine bone collagen peptides prepared by ultrasound-assisted enzymatic hydrolysis. Ultrason Sonochem. 2023;101:106697.
Xiong X-Y, Liang J, Xu Y-Q, Liu Y. The Tilapia collagen peptide mixture TY001 protects against LPS-induced inflammation, disruption of glucose metabolism, and aberrant expression of circadian clock genes in mice. Chronobiol Int. 2019;36:1013–23.
Guo X, Li X, Dong Y, Xie W, Jin T, Xu D, et al. Cod (Gadus) skin collagen peptide powder reduces inflammation, restores mucosal barrier function, and inhibits fibrosis in dextran sodium sulfate-induced colitis in mice. J Ethnopharmacol. 2023;316:116728.
Rahabi M, Salon M, Bruno-Bonnet C, Prat M, Jacquemin G, Benmoussa K, et al. Bioactive fish collagen peptides weaken intestinal inflammation by orienting colonic macrophages phenotype through mannose receptor activation. Eur J Nutr. 2022;61:2051–66.
Zhao W, Li J, Li Y, Chen Y, Jin H. Preventive effect of collagen peptides from Acaudina molpadioides on acute kidney injury through attenuation of oxidative stress and inflammation. Oxid Med Cell Longev. 2022;2022:8186838.
Subhan F, Kang HY, Lim Y, Ikram M, Baek S-Y, Jin S, et al. Fish scale collagen peptides protect against CoCl2/TNF-α-induced cytotoxicity and inflammation via inhibition of ROS, MAPK, and NF-κB pathways in HaCaT Cells. Oxid Med Cell Longev. 2017;2017:9703609.
Yang Y, Zhu L, Guo Z, Liu C, Hu B, Li M, et al. Yak bone collagen-derived anti-inflammatory bioactive peptides alleviate lipopolysaccharide-induced inflammatory by inhibiting the NF-κB signaling pathway and nitric oxide production. Food Biosci. 2023;52:102423.
Bischof K, Stafilidis S, Bundschuh L, Oesser S, Baca A, König D. Reduction in systemic muscle stress markers after exercise-induced muscle damage following concurrent training and supplementation with specific collagen peptides - a randomized controlled trial. Front Nutr. 2024;11:1384112.
Clifford T, Ventress M, Allerton DM, Stansfield S, Tang JCY, Fraser WD, et al. The effects of collagen peptides on muscle damage, inflammation and bone turnover following exercise: a randomized, controlled trial. Amino Acids. 2019;51:691–704.
Wang H. A review of the effects of collagen treatment in clinical studies. Polymers 2021;13:3868.
Barnett ML, Kremer JM, St. Clair EW, Clegg DO, Furst D, Weisman M, et al. Treatment of rheumatoid arthritis with oral type II collagen: results of a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 1998;41:290–7.
Wei W, Zhang L-L, Xu J-H, Xiao F, Bao C-D, Ni L-Q, et al. A multicenter, double-blind, randomized, controlled phase III clinical trial of chicken type II collagen in rheumatoid arthritis. Arthritis Res Ther. 2009;11:R180.
Garcia-Macedo R, Sanchez-Muñoz F, Almanza-Perez JC, Duran-Reyes G, Alarcon-Aguilar F, Cruz M. Glycine increases mRNA adiponectin and diminishes pro-inflammatory adipokines expression in 3T3-L1 cells. Eur J Pharm. 2008;587:317–21.
Luo L, Liu M. Adiponectin: friend or foe in obesity and inflammation. Med Rev. 2022;2:349–62. 2021.
Nguyen TMD. Adiponectin: role in physiology and pathophysiology. Int J Prev Med. 2020;11:136.
Yanai H, Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. Int J Mol Sci. 2019;20:1190.
Chen J, Ma X, Yang Y, Dai Z, Wu Z, Wu G. Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis. Amino Acids. 2018;50:629–40.
Alarcon-Aguilar FJ, Almanza-Perez J, Blancas G, Angeles S, Garcia-Macedo R, Roman R, et al. Glycine regulates the production of pro-inflammatory cytokines in lean and monosodium glutamate-obese mice. Eur J Pharm. 2008;599:152–8.
Aisike G, Kuerbanjiang M, Muheyati D, Zaibibuli K, Lv M-X, Han J. Correlation analysis of obesity phenotypes with leptin and adiponectin. Sci Rep. 2023;13:17718.
Zhao S, Kusminski CM, Scherer PE. Adiponectin, leptin and cardiovascular disorders. Circ Res. 2021;128:136–49.
Wang Y, Nakajima T, Gonzalez FJ, Tanaka N. PPARs as metabolic regulators in the liver: lessons from liver-specific PPAR-null mice. Int J Mol Sci. 2020;21:2061.
Kang M-J, Kim KK, Son BY, Nam S-W, Shin P-G, Kim G-D. The anti-adipogenic activity of a new cultivar, pleurotus eryngii var. ferulae beesan no. 2, through down-regulation of PPAR and C/EBP in 3T3-L1 cells. J Microbiol Biotechnol. 2016;26:1836–44.
Hyun-Jung Lee, Minji Woo, Yeong Ok Song, Jeong Sook Noh. Inhibitory effect of skate skin collagen on hepatic lipid accumulation through regulation of lipid metabolism. J Korean Soc Food Sci Nutr. 2018; 47:235–42.
Almanza-Perez JC, Alarcon-Aguilar FJ, Blancas-Flores G, Campos-Sepulveda AE, Roman-Ramos R, Garcia-Macedo R, et al. Glycine regulates inflammatory markers modifying the energetic balance through PPAR and UCP-2. Biomed Pharmacother. 2010;64:534–40.
Nomura K, Kimira Y, Kobayashi R, Shiobara Y, Osawa Y, Kataoka-Matsushita A, et al. Collagen-derived dipeptide prolyl-hydroxyproline cooperates with Foxg1 to activate the PGC-1α promoter and induce brown adipocyte-like phenotype in rosiglitazone-treated C3H10T1/2 cells. Front Nutr. 2024;11:1375532.
Amabebe E, Robert FO, Agbalalah T, Orubu ESF. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr. 2020;123:1127–37.
Jiang S, Dong W, Zhang Z, Xu J, Li H, Zhang J, et al. A new iron supplement: the chelate of pig skin collagen peptide and Fe2+ can treat iron-deficiency anemia by modulating intestinal flora. Front Nutr. 2022;9:1055725.
Chen W, Zhang M, Guo Y, Wang Z, Liu Q, Yan R, et al. The profile and function of gut microbiota in diabetic nephropathy. Diab Metab Syndr Obes. 2021;14:4283–96.
Salehi S, Hosseinzadeh-Attar MJ, Alipoor E, Dahmardehei M, Yaseri M, Emami MR, et al. Effects of hydrolyzed collagen alone or in combination with fish oil on the gut microbiome in patients with major burns. Burns. 2024;50:444–53.
Hooijmans CR, IntHout J, Ritskes-Hoitinga M, Rovers MM. Meta-analyses of animal studies: an introduction of a valuable instrument to further improve healthcare. ILAR J. 2014;55:418–26.
Leenaars, Kouwenaar CHC, Stafleu C, Bleich FR, Ritskes-Hoitinga A, Vries M, et al. Animal to human translation: a systematic scoping review of reported concordance rates. J Transl Med. 2019;17:223.
Bahadoran Z, Mirmiran P, Kashfi K, Ghasemi A. Importance of systematic reviews and meta-analyses of animal studies: challenges for animal-to-human translation. J Am Assoc Lab Anim Sci. 2020;59:469–77.
U.S. Food and Drug Administration. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers 2005. https://www.fda.gov/media/72309/download.
Oikawa SY, Kamal MJ, Webb EK, McGlory C, Baker SK, Phillips SM. Whey protein but not collagen peptides stimulate acute and longer-term muscle protein synthesis with and without resistance exercise in healthy older women: a randomized controlled trial. Am J Clin Nutr. 2020;111:708–18.
Bischof K, Moitzi AM, Stafilidis S, König D. Impact of collagen peptide supplementation in combination with long-term physical training on strength, musculotendinous remodeling, functional recovery, and body composition in healthy adults: a systematic review with meta-analysis. Sports Med. 2024;54:2865–88.
Wu Y, Deng S, Wei W, He Y, He Y, Hong G, et al. Oral collagen-based supplement as a bioactive component in functional foods. Collagen Leather. 2025;7:1–22.
Du Percie Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020;18:e3000411.
Kousholt BS, Præstegaard KF, Stone JC, Thomsen AF, Johansen TT, Ritskes-Hoitinga M, et al. Reporting quality in preclinical animal experimental research in 2009 and 2018: a nationwide systematic investigation. PLoS ONE. 2022;17:e0275962.
Acknowledgements
We want to thank all authors of the studies included in the current meta-analysis for providing their data upon request.
Funding
Open access funding provided by University of Vienna.
Author information
Authors and Affiliations
Contributions
KB designed the review; AMM and KB performed screening and study quality assessment; KB and AMM analyzed the data; KB wrote the manuscript; AMM, DK, and KB reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bischof, K., Moitzi, A.M. & König, D. Regular collagen peptide administration exerts anti-obesity effects in high-caloric diet-fed rodents—a systematic review with meta-analysis of animal trials. Int J Obes 50, 8–22 (2026). https://doi.org/10.1038/s41366-025-01905-3
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41366-025-01905-3