Abstract
Background
Despite evidence from experimental studies linking some petroleum hydrocarbons to markers of immune suppression, limited epidemiologic research exists on this topic.
Objective
The aim of this cross-sectional study was to examine associations of oil spill related chemicals (benzene, toluene, ethylbenzene, xylene, and n-hexane (BTEX-H)) and total hydrocarbons (THC) with immune-related illnesses as indicators of potential immune suppression.
Methods
Subjects comprised 8601 Deepwater Horizon (DWH) oil spill clean-up and response workers who participated in a home visit (1–3 years after the DWH spill) in the Gulf Long-term Follow-up (GuLF) Study. Cumulative exposures to THC and individual BTEX-H constituents during the oil spill clean-up were estimated using a job-exposure matrix linking air measurement data to detailed participant work histories. Study outcomes included post-spill occurrence and/or frequency of illnesses ascertained at the home visit, including colds, flu, cold sores, pneumonia, and shingles. Frequent cold and frequent flu were defined as ≥4 colds and ≥2 episodes of flu since the spill, respectively. We examined an aggregate outcome of frequent colds, any flu, cold sores, or pneumonia since the spill. In single pollutant models, we used multivariable log-binomial regression to estimate prevalence ratios (PR) and 95% confidence intervals (CI) for associations between quartiles of THC and BTEX-H exposures with each outcome. We used quantile g-computation to estimate the joint effect of the BTEX-H mixture.
Results
We observed positive associations of increasing quartiles of THC and BTEX-H with all outcomes except shingles, with evidence of an exposure-response for most outcomes. Strongest associations were observed for frequent flu (range of PR: 1.41–1.67). The BTEX-H mixture was associated with small to modest elevations in PRs for most outcomes.
Impact Statement
This study is the first to our knowledge to demonstrate an association between oil spill BTEX-H exposures and multiple immune-related illnesses as measures of potential immune suppression. Increasing oil spill-related volatile hydrocarbon exposures may increase the risk of multiple immune-related illnesses, especially frequent cold and frequent flu. Future research on this topic using more robust measures of immune function would advance existing evidence on this relationship.
Similar content being viewed by others
Introduction
The 2010 Deepwater Horizon (DWH) oil spill was the largest marine oil spill in United States (US) history, resulting in the release of nearly 4.9 million barrels of oil into the US Gulf of Mexico [1, 2]. Oil spills not only contaminate the environment and harm ecosystems, but they can also impact the health of response workers [3,4,5,6,7,8,9,10,11,12,13]. Workers who assisted in the clean-up of the DWH oil spill were exposed to a wide range of petrochemicals, such as total hydrocarbons (THC) including benzene, toluene, ethylbenzene, xylene, and n-hexane (BTEX-H). [1, 14,15,16,17,18,19,20,21] Previous epidemiologic studies of oil spill-related exposures have found positive associations with respiratory functions and symptoms such as cough and wheeze, [12, 13, 22,23,24,25] but little research has examined effects of these exposures on the immune system. Petroleum continues to be relied on as a primary source of energy in most of the world and spills of various sizes are common. It is, therefore, vital to examine how oil spill exposures may impact the immune health of workers engaged in the response and clean-up of such spills.
Prior studies on the effects of benzene exposure on markers of immune function have found exposure to be associated with lower levels of several immune markers including immunoglobulin, CD4, IgM and IgA [26, 27]. Other studies have focused on effects of volatile hydrocarbon exposures on potentially immune-mediated respiratory outcomes [12, 13, 22,23,24]. However, these studies grouped and examined immune-related symptoms and illnesses such that effects of volatile hydrocarbon exposures on immune suppression cannot be independently disentangled from inflammation. Further, these studies relied solely on self-reported clean-up work and/or location of residence as proxies of spill-related exposure since they lacked quantitative exposure assessments [20,21,22, 24].
Additional research is needed to elucidate the effects of oil spill exposures on immune-related health. The present study adds to the limited literature on this topic by examining quantitative, chemical-specific oil spill exposures that incorporate air measurement data as well as individual immune-related illnesses suggestive of potential immune suppression [17]. The objective of this study is to estimate the association between exposure to oil spill clean-up-related THC and BTEX-H and multiple immune-related illnesses among OSRC workers following the DWH disaster.
Methods
Study population
The GuLF Study is a prospective cohort of 32,608 adults who were ≥21 years of age at enrollment, which occurred from March 2011 to May 2013 [17]. Participants in the cohort included both DWH oil spill response and clean-up (OSRC) workers and non-workers. Individuals were eligible for study enrollment if they performed at least one day of OSRC work on the DWH oil spill (workers), which began on April 20th, 2010, or received oil spill worker safety training but did not work on the spill (non-workers). Study enrollment was conducted via computer-assisted telephone interviews which collected detailed data on spill-related response and clean-up work tasks and activities and demographic, lifestyle, and health factors. All English and Spanish speaking study participants from eastern Texas, Louisiana, Mississippi, Alabama, and Florida were invited to participate in a home visit 1–3 years after the spill, from May 2011 to May 2013. Of the 25,304 individuals who were eligible, 7421 declined; 4528 were lost to follow-up; 2137 changed their minds after initially agreeing to participate; and 25 did not complete the visit for health or safety reasons, resulting in a total of 11,193 home visit participants.
Since exposure estimates are available only for OSRC workers, and health data of interest were collected during a home visit survey, we restricted the present analyses to OSRC workers who participated in the home visit (N = 8968). We excluded those missing THC or BTEX-H exposure estimates or study covariates, for a final population of 8601.
Exposure assessment
Cumulative average exposure to THC (ppm-days) and BTEX-H (ppb-days) was estimated via a job-exposure matrix that linked air measurement data to the self-reported OSRC work history of each participant. [1, 15,16,17,18,19, 28,29,30,31,32] Air measurement data came from over 28,000 air samples collected using organic vapor passive dosimeters on a subset of workers during OSRC work between April 2010 and June 2011 [19, 28]. Laboratory analysis (using NIOSH method 1501 and OSHA 7 method) of these samples resulted in over 143,000 measurements of THC and individual BTEX-H constituents [28]. For days when there were few or no personal measurements on particular vessels, study industrial hygienists converted 26 million direct-reading volatile organic compound (VOC) area measurements to the equivalent full-shift THC and BTEX-H estimates [1, 31]. Multigas detectors (AreaRAE and MultiRAE) equipped with a photoionizing detector lamp collected these direct-reading VOC area measurements from 38 large vessels [29, 31].
Industrial hygienists estimated exposures for each study participant after creating over 3000 exposure groups (EGs) based on three determinants: self-reported work job/activity (e.g., skimming water to collect surface oil, burning surface oil); location of work (four Gulf coastal states (Louisiana, Mississippi, Alabama, Florida) and hot zone, source, offshore, near-shore, and land); and time period (seven time periods over 14 months that captured changes in OSRC events and oil weathering) [15, 16, 18, 32]. The geometric mean of the corresponding air measurements was estimated for each EG [19]. Each EG comprised individuals who were expected to have performed similar OSRC tasks, based on their self-reported OSRC work history, and to have similar exposure distributions [18]. For EGs with a large number of measurements below the limit of detection (LOD), industrial hygienists used a left-censored Bayesian framework to estimate exposure averages [1, 31]. In the current analysis, we summarized the THC and BTEX-H exposure as the cumulative daily average (the daily average exposure estimates across all jobs/activities a worker performed in a day, summed across all days within a time period and then across all time periods) [1, 16, 18, 32]. These cumulative exposure estimates were calculated to assess the total burden of exposure experienced during clean-up. A more detailed explanation of the exposure assessment is further described elsewhere [1, 30].
Health outcome assessment
Outcomes in this study, as indirect measures of potential immune suppression, were occurrence and/or frequency of colds, flu, cold sores, pneumonia, and shingles self-reported in a structured interview during the home visit. During the interview, participants were asked about the occurrence and/or frequency of these illnesses in the previous 1–3 years since the DWH oil spill. The number of years since the DWH oil spill depended upon the timing of each participant’s home visit, which occurred 1–3 years after the spill. Participants were asked, separately, whether they had ever had a cold or the flu in the previous 1–3 years (i.e., since the oil spill); those who responded “yes” were asked about the number of colds or flu episodes experienced during that 1–3 year period. For cold sores, participants were first asked whether they had ever had cold sores or fever blisters on their lips; those who responded “yes” were then asked whether they had at least one episode in the previous 1–3 years since the oil spill. For pneumonia and shingles, participants were asked separately whether they had ever had the condition; those who responded “yes” were then asked whether they had seen a doctor for that condition in the previous 1–3 years since the oil spill. Individuals who reported not having experienced that condition during the 1–3 year period were categorized as never experiencing the condition (during the observation period).
Potential confounders
The GuLF Study enrollment and home visit interviews collected extensive information on potential confounders. A directed acyclic graph (DAG) was used to determine a minimally sufficient adjustment set for assessing the association of THC and BTEX-H with each outcome [33]. Variables considered included age, sex, self-identified race, Hispanic ethnicity, educational attainment, annual household income, employment status, physical activity, body mass index (BMI), smoking, alcohol consumption, secondhand smoke, high stress (based on Cohen’s Perceived Stress Scale score ≥9) [34], previous oil spill-related clean-up work or oil industry experience, physician diagnosis of diabetes prior to the oil spill, and residential proximity to the oil spill. All covariates came from the enrollment interview except age, which was calculated for the home visit. Race and ethnicity are considered potential confounders since they may have influenced exposure opportunity to oil spill clean-up related compounds and immune-related illnesses due to residence, housing, and other factors driven by structural racism [35,36,37].
Statistical analysis
Descriptive statistics of THC and BTEX-H exposures, the immune-related illnesses, and covariates were examined via counts and percentages. Cumulative exposures to THC and BTEX-H were categorized into quartiles to assess any non-linear exposure-response relationships (Supplementary Table 1). Specific outcomes in this study included the following potentially immune-related illnesses since the spill: any colds, any flu episodes, cold sores, pneumonia, and shingles. We further created measures of frequent colds and frequent flu, defined as ≥4 colds and ≥2 episodes of flu since the spill, respectively. In addition, we defined an aggregate outcome as occurrence of pneumonia, cold sores, any flu, or frequent colds since the spill. We used multivariable log-binomial regression to estimate prevalence ratios (PR) and 95% confidence intervals (CI) for associations of THC and BTEX-H quartiles with each outcome (except shingles); the referent group comprised subjects in the lowest quartile of the given exposure. For shingles, because of the small number of cases, we dichotomized exposures, comparing those in the combined top three quartiles (high exposure) to those in the lowest quartile (low exposure). We also used quantile g-computation to estimate the joint effect of the BTEX-H mixture on each outcome using R package version 2.9.0 “qgcomp” [38].
In the multivariable models for cold, flu, and cold sores, we adjusted for age (continuous), race (White, Black, other), ethnicity (Hispanic, not Hispanic), sex (male, female), residential proximity to the oil spill (living in a county or Parish directly adjacent to the Gulf of Mexico, a county/Parish adjacent to those coastal counties, a Gulf or non-Gulf state further from the spill), highest educational attainment (less than high school, high school diploma/General Education Development, some college/2-year degree, 4-year college graduate or more), physician diagnosis of diabetes prior to the oil spill (yes, no), oil industry experience prior to the oil spill (yes, no), and years between the oil spill and the home visit (continuous). We adjusted for time elapsed between the spill and home visit, which ranged from 1 to 3 years, to account for potential differential recall or reporting of outcomes and variation in the opportunity to experience these immune-related illnesses. The “other” race category combined those who self-identified as Asian (0.53%), other (6.5%), or multi-racial (2.8%) due to small sample sizes within these groups. Due to the relatively small number of pneumonia and shingles cases, the adjustment set for pneumonia consisted of age at the home visit, sex, residential proximity to the oil spill, and years between oil spill and home visit, and for shingles consisted of age at the home visit and years between oil spill and home visit. We considered these covariates as the most important to adjust for confounding for pneumonia and shingles. We conducted sensitivity analyses to assess any uncontrolled confounding by covariates included in adjusted analyses for cold, flu, and cold sores but not in analyses for the reduced models for pneumonia and shingles. Specifically, we added each confounder individually to the multivariable models used in pneumonia and shingles analyses and compared the resultant effect estimates to the original estimate from the reduced model.
Multiplicative interaction terms were used to assess effect measure modification (EMM) by age ( < 40, 40–59, ≥60 years), sex (male, female), receipt of flu shot since the spill (yes, no), season of home visit (fall/winter, spring/summer), and number of children <18 years of age living at home at the time of the home visit (0, ≥1). For modification of associations with the BTEX-H mixture, we used stratified models to evaluate effects within subgroups. We selected the age categories based on sample sizes within each group and potential variation in risk of immune-related illness by age. EMM analyses by self-reported receipt of flu shot, season of home visit interview, and number of children were specific to relevant outcomes: flu outcomes were stratified by receipt of flu shot; cold and flu outcomes were stratified by season of home visit interview; and cold, flu, and cold sore outcomes were stratified by number of children <18 years of age living at home. There were insufficient shingles cases for EMM analyses.
Results
The average age of participants at the home visit was 43 years (Table 1). The majority of participants were male (80%), White (55%), and had either high school (34%) or less than high school (21%) educational attainment. 55% of participants self-reported as White, 35% as Black, and 10% as other race. Only 6% identified as Hispanic. Most participants reported residing directly adjacent to the Gulf of Mexico (73%) at enrollment and no prior oil industry work experience (83%) nor prior oil spill clean-up experience (92%). The most prevalent outcome was any cold (73%), followed by frequent cold (24%), cold sores (21%), and any flu (18%). Only 7% of participants reported frequent flu between the oil spill and home visit interview. Nearly half of participants had the aggregate outcome (48%). Few participants reported occurrence of pneumonia (3%) or shingles (1%) since the spill. The THC and BTEX-H exposures were strongly correlated (r = 0.84–0.95) (Supplementary Table 2).
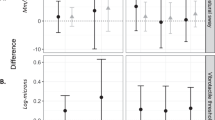
Increasing quartiles of THC and BTEX-H cumulative average exposures were positively associated with prevalence of most outcomes, with many that demonstrated evidence of exposure-response trends (Fig. 1, Supplementary Table 3). Only shingles showed no significant association with any of the exposures; however, shingles was relatively rare among subjects, resulting in imprecise effect estimates and limiting our ability to assess exposure-response relationships. Crude and adjusted effect estimates were similar (crude results not shown). Across all exposures, stronger associations were observed for frequent colds and frequent flu compared to any colds and any flu, respectively. For example, the highest quartile of THC was associated with a PR of 1.51 (95% CI: 1.35, 1.69, p-trend: <0.01) for frequent colds and a PR of 1.67 (95% CI: 1.32, 2.11, p-trend: <0.01) for frequent flu, compared to a PR of 1.07 (95% CI: 1.03, 1.11, p-trend: <0.01) for any colds and a PR of 1.32 (95% CI: 1.15, 1.51, p-trend: <0.01) for any flu. We observed a monotonic increase in prevalence of most outcomes across exposure quartiles for THC and ethylbenzene; results were largely similar for xylene and n-hexane. The strongest associations were generally observed for frequent flu, with PRs for the highest versus lowest quartile of exposure ranging from 1.41 (95% CI: 1.11, 1.79, p-trend: 0.03) for toluene to 1.67 (95% CI: 1.32, 2.11, p-trend: <0.01) for THC. Sensitivity analyses to assess any uncontrolled confounding in the reduced adjustment sets for pneumonia and shingles did not demonstrate meaningful changes in the effect estimates and 95% CIs (results not shown). Additionally, results were not substantively different when we stratified by time elapsed between the oil spill and home visit (2 years or 3 years). Models did not converge for those whose time elapsed between the oil spill and home visit was 1 year, due to small sample sizes. In analyses using THC and BTEX-H exposures as the cumulative daily maximum (the sum of daily maximum exposure estimates across all days within a time period and then across all time periods), we observed associations similar (results not shown) to those for cumulative average exposures.
When we assessed the joint effect of the BTEX-H mixture using quantile g-computation (Table S4), we found that a one quartile simultaneous increase in cumulative daily average exposure to each component of the mixture was associated with small to modest elevations in PRs for most outcomes, ranging from 1.02 for any cold (95% CI: 1.01, 1.04) to 1.16 for frequent flu (95% CI: 1.08, 1.25).
Stratified analyses of THC associations suggested EMM by sex and age (Figs. 2 and 3). We found modestly higher prevalence ratios and more consistent exposure-response trends for THC in relation to frequent colds, frequent flu, and the aggregate outcome among males compared to females, although there were substantially more males than females in the cohort and confidence intervals generally overlapped (Fig. 2). We observed similar patterns among males and females for the BTEX-H exposures in relation to these outcomes (Table S5; Figs. S1-S5). Prevalence ratios were slightly higher across THC and BTEX-H exposures for any colds and cold sores among females compared to males, although we did not consistently observe exposure-response trends in these analyses. For the BTEX-H mixture, we only found slightly stronger associations among females for any colds and pneumonia (Table S5, Fig. S16). When stratified by age, prevalence ratios for the effect of THC and BTEX-H on frequent colds were modestly higher among those aged 40–59 than those <40 years of age (Fig. 3; Table S6; Figs. S6-S10). Confidence intervals for prevalence ratios among those ≥60 years were generally wide due to the smaller sample size of this age group. Nonetheless, among those aged ≥60 years, associations for frequent flu appeared stronger compared to the other age groups. We did not observe substantively different associations in the ≥60 years age group for the other outcomes. While associations of THC and BTEX-H with any cold were slightly elevated for those aged 40–59 years, associations were generally weaker or absent among those <40 or ≥60 years of age. We found slightly higher prevalence ratios for the association of the BTEX-H mixture with frequent cold among those aged 40–59 years compared to the other age groups and with frequent flu among those aged <40 years compared to those aged 40–59 years (Fig. S16).
The association of exposure to THC and the BTEX-H mixture with any flu and frequent flu were modestly stronger among those who had received a flu shot since the spill compared to those who had not (Figs. 4 and S16). This difference was still apparent, but less pronounced, for the individual BTEX-H exposures (Table S7; Figs. S11-S15). We found no apparent modification of associations of THC and BTEX-H with most outcomes by the presence of children aged <18 years residing at home (Table S8) or season of home visit (Table S9 and Fig. S17). However, for the BTEX-H mixture we observed slightly stronger associations for frequent cold among those without children <18 years and for frequent flu among those with children <18 years (Table S8 and Fig. S17).
Discussion
We found cumulative THC, individual BTEX-H components, and the BTEX-H mixture exposures during oil spill clean-up to be positively associated with occurrence and/or frequency of colds, flu, cold sores, and pneumonia among Deepwater Horizon oil spill response and clean-up workers 1–3 years after the spill. Of note, THC exposure, an indicator of overall crude oil inhalation exposure during clean-up, exhibited monotonic exposure-response relationships with most of the study outcomes. Further, we found suggestive evidence of EMM by age and sex, with higher prevalence ratios among those who were 40–59 years of age and males. Results of sensitivity analyses were similar to those of the main analyses, increasing confidence in these findings.
Our findings align with the limited prior research on the association between volatile hydrocarbon exposures and markers of immune suppression [26, 27]. A cross-sectional study was conducted in India among 428 gasoline filling workers and 78 non-workers matched to the gasoline filling workers on socioeconomic status, age, and sex [26] The investigators reported significantly lower blood counts/levels of the immune markers CD4, CD4/CD8 ratio, IgG1, and IgG2 among the gasoline filling workers compared to the non-workers; however, no significant difference was observed in CD8 count [26]. Another study reported lower serum concentrations of IgM, IgA, and CD4 T cells among 10 exposed workers who maintained cargo tanks containing crude oil residues versus 9 unexposed workers, after adjusting for age, sex, and current smoking [27]. Both of these studies had relatively small sample sizes and lacked sufficient adjustment for confounders [26, 27]. While our study does not utilize biological markers of immune suppression, our study builds upon existing literature using the large GuLF Study cohort with detailed information on covariates and air measurement data and considers immune-related outcomes that are related to quality of life. Additionally, our study found that oil spill THC and BTEX-H exposure estimates were associated with multiple immune-related illnesses even though THC and BTEX-H exposure estimates among the GuLF Study cleanup workers were below contemporaneous occupational exposure limits by the Occupational Safety and Health Administration and the American Conference of Governmental Industrial Hygienists [15, 32].
The mechanisms by which individual BTEX-H exposures affect immune function have been investigated in experimental human and animal studies [39,40,41,42,43,44,45,46,47]. Benzene, ethylbenzene, and n-hexane may suppress immune function via decreases in serum immunoglobulins in humans [39, 43]. Animal studies have demonstrated immunosuppressive effects of benzene and toluene by a reduction in B and T lymphocytes [45,46,47]. However, less evidence exists in human studies for potential mechanisms by which toluene and xylene exposure may affect immune health [40, 42]. The broad generalizability of results from these studies are limited due to potential modification of effects by other exposures that often co-occur with BTEX-H (and that may be present in THC), differences in study design, and biological differences between animals and humans [39,40,41,42,43,44,45,46,47].
The current study has several limitations. First, a limitation for the exposure assessment is the possibility that participants may not have accurately reported their OSRC work history, leading to misclassification. Second, the illnesses that we used as markers of potential immune suppression are subject to recall bias and non-differential misclassification with respect to oil spill clean-up related exposures, especially for colds, flu, and cold sores since these outcomes are more common. Third, the amount of time elapsed between the DWH spill and the home visit (when these outcomes were ascertained) ranged from 1–3 years, which may have led to differential recall or reporting of outcomes. To address this, we stratified by the number of years between the spill and home visit in sensitivity analyses and did not observe meaningful differences in results compared to the main analysis where we adjusted for this variation in time elapsed between the spill and home visit. Fourth, although receipt of flu shots and episodes of flu were ascertained for the period between the spill and the home visit, we lacked information on the timing of any flu shots relative to episodes of flu. We assumed that receipt of a flu shot reflected generally more healthful behavior and took place in the recommended time window or perhaps having had the flu motivated the respondent to get a flu shot. The modestly higher prevalence ratios for flu outcomes among those who received a flu shot (versus no flu shot) may be partially explained by residual confounding: flu shot recipients tended to be older in age and had prevalent diabetes prior to the oil spill, although we did adjust for these factors. These individuals may have been more likely to receive a flu shot due to decreased immune function associated with older age and diabetes. Fifth, generally similar associations observed across the BTEX-H exposures may be due in part to co-pollutant confounding. Sixth, risk estimates were relatively imprecise in some subgroups (e.g., women, those aged ≥ 60 years) because of the small sizes of those subgroups. Seventh, we were unable to conduct analyses excluding individuals with autoimmune conditions (e.g., rheumatoid arthritis, lupus, and Graves) or who used immunosuppressants. This was due to substantial missingness in reporting of these conditions. However, we do not expect this to substantively impact the observed risk estimates given that these autoimmune conditions are relatively rare [48,49,50] and few individuals reported use of immunosuppressants (N = 48). Eighth, there may be a bias if workers were assigned to jobs and activities based on certain health factors (healthy worker effect). We minimized this potential bias by adjusting for physician diagnosis of diabetes prior to the oil spill as an indicator of baseline health. However, a bias may persist with healthier workers experiencing higher exposures, which potentially led to an underestimation of the associations observed in our study.
Strengths of our study include use of the prospective GuLF Study cohort, which is the largest study to date of oil spill clean-up and response workers. Moreover, while most previous studies of oil spill-exposed populations used relatively crude proxies of exposure, such as self-reported days of work and/or location of residence, exposures in our study were estimated via a job-exposure matrix based on extensive air measurements collected during the spill response. Cumulative exposure estimates were derived from personal air samples used by a subset of OSRC workers during the DWH spill and were linked to detailed DWH clean-up work histories collected from each study participant. Our study leveraged occurrence of multiple immune-related illnesses as proxies of potential immune suppression in relation to oil spill-related THC and BTEX-H exposures. Finally, we were able to adjust for a range of important confounders and assess several potential effect measure modifiers in our analysis due to the extensive covariate data collected from the GuLF Study cohort.
This study is the first to demonstrate an association between oil spill BTEX-H exposures and multiple immune-related illnesses as measures of potential immune suppression. We found evidence of stronger associations for those aged 40–59 years and for males. These findings are particularly noteworthy given that estimated BTEX-H exposures among our GuLF Study participants were below exposure limits set by the Occupational Safety and Health Administration and American Conference of Governmental Industrial Hygienists for THC and BTEX-H respectively, [16, 32, 39,40,41,42,43,44] but higher than those typically observed in the general US population [51]. Future research in this area would benefit from additional measures of immune function, including molecular markers, ideally captured at multiple time points both before and after exposure.
Data availability
The authors are prohibited from making the data set publicly available, however, study proposals may be submitted to the GuLF Study via EpiShare. Collaborators interested in conducting research using the GuLF Study may contact the study at info@gulfstudy.nih.gov to find out if work you propose is not already underway.
References
Groth C, Banerjee S, Ramachandran G, Stenzel MR, Sandler DP, Blair A, et al. Bivariate Left-Censored Bayesian Model for Predicting Exposure: Preliminary Analysis of Worker Exposure during the Deepwater Horizon Oil Spill. Ann Work Exposures Health. 2017;61:76–86.
National Commission on the BP Deepwater Horizon Oil Spill and Offshore Drilling. Deep water: the Gulf oil disaster and the future of offshore drilling: report to the President. Washington, D.C.: National Commission on the BP Deepwater Horizon Oil Spill and Offshore Drilling: For sale by the Supt. of Docs., U.S. G.P.O; 2011. 380.
Aguilera F, Méndez J, Pásaro E, Laffon B. Review on the effects of exposure to spilled oils on human health. J Appl Toxicol. 2010;30:291–301.
Cheong HK, Ha M, Lee JS, Kwon H, Ha EH, Hong YC, et al. Hebei Spirit Oil Spill Exposure and Subjective Symptoms in Residents Participating in Clean-Up Activities. Environ Health Toxicol. 2011;26:e2011007.
Fan AZ, Prescott MR, Zhao G, Gotway CA, Galea S. Individual and Community-Level Determinants of Mental and Physical Health After the Deepwater Horizon Oil Spill: Findings from the Gulf States Population Survey. J Behav Health Serv Res. 2015;42:23–41.
Janjua NZ, Kasi PM, Nawaz H, Farooqui SZ, Khuwaja UB, Najam-ul-Hassan, et al. Acute health effects of the Tasman Spirit oil spill on residents of Karachi, Pakistan. BMC Public Health. 2006;6:84.
Kim BM, Park EK, LeeAn SY, Ha M, Kim EJ, Kwon H, et al. BTEX Exposure and its Health Effects in Pregnant Women Following the Hebei Spirit Oil Spill. J Preventive Med Public Health. 2009;42:96–103.
Lowe SR, McGrath JA, Young MN, Kwok RK, Engel LS, Galea S, et al. Cumulative Disaster Exposure and Mental and Physical Health Symptoms Among a Large Sample of Gulf Coast Residents. J Trauma Stress. 2019;32:196–205.
Meo SA, Al-Drees AM, Rasheed S, Mu Meo I, Al-Saadi MM, Ghani HA, et al. Health Complaints Among Subjects Involved in Oil Cleanup Operations During Oil Spillage from a Greek Tanker “Tasman Spirit”. Int J Occup Med Environ Health. 2009;22:143–8.
Na JU, Sim MS, Jo IJ, Song HG. The duration of acute health problems in people involved with the cleanup operation of the Hebei Spirit oil spill. Mar Pollut Bull. 2012;64:1246–51.
Park MS, Choi KH, Lee SH, Hur JI, Noh SR, Jeong WC, et al. Health effect research on Hebei Spirit Oil Spill (HEROS) in Korea: a cohort profile. BMJ Open. 2019;9:e026740.
Zock JP, Rodríguez-Trigo G, Pozo-Rodríguez F, Barberà JA, Bouso L, Torralba Y, et al. Prolonged Respiratory Symptoms in Clean-up Workers of the Prestige Oil Spill. Am J Respir Crit Care Med. 2007;176:610–6.
Zock JP, Rodríguez-Trigo G, Rodríguez-Rodríguez E, Espinosa A, Pozo-Rodríguez F, Gómez F, et al. Persistent respiratory symptoms in clean-up workers 5 years after the Prestige oil spill. Occup Environ Med. 2012;69:508–13.
Goldstein BD, Osofsky HJ, Lichtveld MY. The Gulf Oil Spill. N. Engl J Med. 2011;364:1334–48.
Huynh TB, Groth CP, Ramachandran G, Banerjee S, Stenzel M, Blair A, et al. Estimates of Inhalation Exposures to Oil-Related Components on the Supporting Vessels During the Deepwater Horizon Oil Spill. Ann Work Exposures Health. 2022;66:i111–23.
Huynh TB, Groth CP, Ramachandran G, Banerjee S, Stenzel M, Blair A, et al. Estimates of Inhalation Exposures among Land Workers during the Deepwater Horizon Oil Spill Clean-up Operations. Ann Work Exposures Health. 2022;66:i124–39.
Kwok RK, Engel LS, Miller AK, Blair A, Curry MD, Jackson WB, et al. The GuLF STUDY: A Prospective Study of Persons Involved in the Deepwater Horizon Oil Spill Response and Clean-Up. Environ Health Perspect. 2017;125:570–8.
Stenzel MR, Groth CP, Huynh TB, Ramachandran G, Banerjee S, Kwok RK, et al. Exposure Group Development in Support of the NIEHS GuLF Study. Ann Work Exposures Health. 2022;66:i23–55.
Stewart P, Groth CP, Huynh TB, Gorman Ng M, Pratt GC, Arnold SF, et al. Assessing Exposures from the Deepwater Horizon Oil Spill Response and Clean-up. Ann Work Exposures Health. 2022;66:i3–22.
Stewart PA, Stenzel MR, Ramachandran G, Banerjee S, Huynh T, Groth C, et al. Development of a Total Hydrocarbon Ordinal Job-Exposure Matrix for Workers Responding to the Deepwater Horizon Disaster: The GuLF STUDY. J Expo Sci Environ Epidemiol. 2018;28:223–30.
Middlebrook AM, Murphy DM, Ahmadov R, Atlas EL, Bahreini R, Blake DR, et al. Air quality implications of the Deepwater Horizon oil spill. Proc Natl Acad Sci USA. 2012;109:20280–5.
Peres LC, Trapido E, Rung AL, Harrington DJ, Oral E, Fang Z, et al. The Deepwater Horizon Oil Spill and Physical Health among Adult Women in Southern Louisiana: The Women and Their Children’s Health (WaTCH) Study. Environ Health Perspect. 2016;124:1208–13.
Rusconi F, Catelan D, Accetta G, Peluso M, Pistelli R, Barbone F, et al. Asthma Symptoms, Lung Function, and Markers of Oxidative Stress and Inflammation in Children Exposed to Oil Refinery Pollution. J Asthma. 2011;48:84–90.
Noh SR, Kim JA, Cheong HK, Ha M, Jee YK, Park MS, et al. Hebei Spirit oil spill and its long-term effect on children’s asthma symptoms. Environ Pollut. 2019;248:286–94.
Gam KB, Kwok RK, Engel LS, Curry MD, Stewart PA, Stenzel MR, et al. Lung function in oil spill response workers 1–3 years after the Deepwater Horizon disaster. Epidemiology. 2018;29:315–22.
Uzma N, Kumar BS, Hazari MAH. Exposure to benzene induces oxidative stress, alters the immune response and expression of p53 in gasoline filling workers. Am J Ind Med. 2010;53:1264–70.
Kirkeleit J, Ulvestad E, Riise T, Bråtveit M, Moen BE. Acute Suppression of Serum IgM and IgA in Tank Workers Exposed to Benzene. Scand J Immunol. 2006;64:690–8.
Stenzel MR, Groth CP, Banerjee S, Ramachandran G, Kwok RK, Engel LS, et al. Exposure Assessment Techniques Applied to the Highly Censored Deepwater Horizon Gulf Oil Spill Personal Measurements. Ann Work Exposures Health. 2022;66:i56–70.
Groth CP, Banerjee S, Ramachandran G, Stewart PA, Sandler DP, Blair A, et al. Methods for the Analysis of 26 Million VOC Area Measurements during the Deepwater Horizon Oil Spill Clean-up. Ann Work Exposures Health. 2022;66:i140–55.
Groth CP, Huynh TB, Banerjee S, Ramachandran G, Stewart PA, Quick H, et al. Linear Relationships Between Total Hydrocarbons and Benzene, Toluene, Ethylbenzene, Xylene, and n-Hexane during the Deepwater Horizon Response and Clean-up. Ann Work Exposures Health. 2022;66:i71–88.
Ramachandran G, Groth CP, Huynh TB, Banerjee S, Stewart PA, Engel LS, et al. Using Real-Time Area VOC Measurements to Estimate Total Hydrocarbons Exposures to Workers Involved in the Deepwater Horizon Oil Spill. Ann Work Exposures Health. 2022;66:i156–71.
Huynh TB, Groth CP, Ramachandran G, Banerjee S, Stenzel M, Quick H, et al. Estimates of Occupational Inhalation Exposures to Six Oil-Related Compounds on the Four Rig Vessels Responding to the Deepwater Horizon Oil Spill. Ann Work Exposures Health. 2022;66:i89–110.
Greenland S, Pearl J, Robins JM. Causal Diagrams for Epidemiologic Research. Epidemiology. 1999;10:37–48.
Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24:385–96.
Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet. 2017;389:1453–63.
Cogburn CD. Culture, Race, and Health: Implications for Racial Inequities and Population Health. Milbank Q. 2019;97:736–61.
Howe CJ, Bailey ZD, Raifman JR, Jackson JW. Recommendations for Using Causal Diagrams to Study Racial Health Disparities. Am J Epidemiol. 2022.10.1093/aje/kwac140.
Keil AP, Buckley JP, O’Brien KM, Ferguson KK, Zhao S, White AJ. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect. 2020;128:047004.
Agency for Toxic Substances and Disease Registry. Toxicological Profile for Benzene. 2024;438. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=40&tid=14.
Agency for Toxic Substances and Disease Registry. Toxicological Profile for Toluene. 2017;496. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=161&tid=29.
Agency for Toxic Substances and Disease Registry. Toxicological Profile for Ethylbenzene. 2010;341. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=383&tid=66.
Agency for Toxic Substances and Disease Registry. Toxicological Profile for Xylene. 2007;385. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=296&tid=53.
Agency for Toxic Substances and Disease Registry. Toxicological Profile for n-Hexane. Accessed 2022 Jul 7. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=393&tid=68.
Agency for Toxic Substances and Disease Registry. Toxicological Profile for Total Petroleum Hydrocarbons. Accessed 2022 Jul 7. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=424&tid=75.
Bracken MB. Why animal studies are often poor predictors of human reactions to exposure. J R Soc Med. 2009;102:120–2.
Veraldi A, Costantini AS, Bolejack V, Miligi L, Vineis P, van Loveren H. Immunotoxic effects of chemicals: A matrix for occupational and environmental epidemiological studies. Am J Ind Med. 2006;49:1046–55.
Bahadar H, Mostafalou S, Abdollahi M. Current understandings and perspectives on non-cancer health effects of benzene: A global concern. Toxicol Appl Pharmacol. 2014;276:83–94.
Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol Int. 2017;37:1551–7.
Hussain YS, Hookham JC, Allahabadia A, Balasubramanian SP. Epidemiology, management and outcomes of Graves’ disease—real life data. Endocrine. 2017;56:568–78.
Izmirly PM, Parton H, Wang L, McCune WJ, Lim SS, Drenkard C, et al. Prevalence of Systemic Lupus Erythematosus in the United States: Estimates From a Meta-Analysis of the Centers for Disease Control and Prevention National Lupus Registries. Arthritis Rheumatol 2021;73:991–6.
Environmental Protection Agency. An Overview of Methods for EPA’s National-Scale Air Toxics Assessment. Office of Air Quality Planning and Standards, Research Triangle Park, NC. 2011. https://www.epa.gov/sites/default/files/2015-10/documents/2005-nata-tmd.pdf.
Acknowledgements
We acknowledge the support of the GuLF STUDY Scientific Advisory Board, Community Advisory Group, and numerous federal, state, and local agencies, and community groups. The NIEHS Office of Human Research Compliance staff (J. Packenham, J. Lambert, and C. Wladyka) and D. Resnik, IRB Chair, facilitated expedited review of study materials. J. Anderson provided oversight as liaison to the NIH Office of the Director. We also thank all former and current GuLF STUDY staff at SRA and Social & Scientific Systems, Inc., and study participants.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, the National Institute of Environmental Health Sciences (Z01 ES 102945).
Author information
Authors and Affiliations
Contributions
Opal P. Patel: formal analysis, software, writing—original draft, visualization. Kaitlyn G. Lawrence: writing—review and editing. Christine G. Parks: writing—review and editing. Patricia A. Stewart: writing—review and editing. Mark R. Stenzel: writing—review and editing. Caroline P. Groth: writing—review and editing. Gurumurthy Ramachandran: writing—review and editing. Sudipto Banerjee: writing—review and editing. Tran B. Huynh: writing—review and editing. Braxton Jackson: software, validation, writing—review and editing. Dale P. Sandler: supervision, writing—review and editing. Lawrence S. Engel: conceptualization, supervision, writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Institutional Review Boards of both the National Institute of Environmental Health Sciences and the University of North Carolina.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patel, O.P., Lawrence, K.G., Parks, C.G. et al. Volatile hydrocarbon exposures and immune-related illnesses among Deepwater Horizon oil spill workers. J Expo Sci Environ Epidemiol 36, 24–32 (2026). https://doi.org/10.1038/s41370-024-00738-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41370-024-00738-y