Abstract
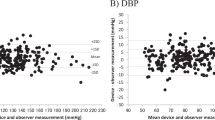
This study evaluated the accuracy of the Hanvon KSY8600 blood pressure monitor in adult populations in accordance with the AAMI/ESH/ISO (ISO 81060-2:2018) and AMD 1:2020 standards. 90 people were recruited as research participants, among which 88 eligible participants were chosen and analyzed according to the research plan. The average age of these participants was 51.3 ± 17.35 years old, with an average arm circumference of 28.1 ± 3.41 cm, and with females accounting for 60.2%. The test results showed that the mean differences (standard deviations) in systolic blood pressure (SBP) and diastolic blood pressure (DBP) displayed on the blood pressure monitor were -0.4 mmHg (2.09 mmHg) and −0.3 mmHg (1.86 mmHg) respectively, meeting the requirements of Criterion 1 (≤ ± 5 mmHg (8 mmHg)). Additionally, the average standard deviations of the participants’ SBP and DBP were 0.91 mmHg and 0.90 mmHg respectively. According to Table 1 of Criterion 2, the mean values of SBP and DBP were −0.4 mmHg and −0.3 mmHg respectively, with maximum allowable standard deviations of SBP and DBP of ≤6.93 and ≤6.95 mmHg respectively, meeting the requirements of Criterion 2. It was thus proved that the blood pressure monitor complies with ISO 81060-2:2018 + AMD1:2020, and is recommended for clinical and home blood pressure measurement for adults.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Analyses and generated datasets that support the current study are not available publicly. The datasets are available from the corresponding author on reasonable request.
References
STRIDE BP. Validated blood pressure monitors. 2024. https://www.stridebp.org/bp-monitors/.
Sharman JE, Tan I, Stergiou GS, Lombardi C, Saladini F, Butlin M, et al. Automated ‘oscillometric’ blood pressure measuring devices: how they work and what they measure. J Hum Hypertens. 2023;37:93–100.
Stergiou GS, Lourida P, Tzamouranis D, Baibas NM. Unreliable oscillometric blood pressure measurement: prevalence, repeatability and characteristics of the phenomenon. J Hum Hypertens. 2009;23:794–800.
Babbs CF. The origin of Korotkoff sounds and the accuracy of auscultatory blood pressure measurements. J Am Soc Hypertens. 2015;9:935–50.
ISO 81060-2:2018/AMD 2:2024 Non-invasive sphygmomanometers — Part 2: Clinical investigation of intermittent automated measurement type — Amendment 2. International Organization for Standardization. 2024. https://www.iso.org/standard/83598.html.
Acknowledgements
This study was funded by Beijing Hanvon Health Technology Co., Ltd.
Funding
This study was funded by Beijing Hanvon Health Technology Co., Ltd., the manufacturer of the Hanvon KSY8600 device. The sponsor had no role in the study design, data analysis, interpretation, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
JW: Conceptualization, Methodology, Investigation, Writing – Original Draft, Writing – Review & Editing, Supervision, Project Administration. YL: Conceptualization, Resources. CC: Investigation, Formal Analysis, Data Curation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the Ethics Committee of the Beijing Geriatric Hospital, and all participants provided written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Liu, Y. & Chen, C. Validation of the hanvon KSY8600 blood pressure monitor in the general population based on ISO 81060-2:2018 + AMD1:2020 protocol. J Hum Hypertens (2026). https://doi.org/10.1038/s41371-026-01111-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41371-026-01111-2