Abstract
C-reactive protein (CRP) is an acute-phase protein that is used as an established biomarker to follow disease severity and progression in a plethora of inflammatory diseases. However, its pathophysiologic mechanisms of action are still poorly defined and remain elusive. CRP, in its pentameric form, exhibits weak anti-inflammatory activity. On the contrary, the monomeric isoform (mCRP) exhibits potent pro-inflammatory properties in endothelial cells, leukocytes, and platelets. So far, no data exists regarding mCRP effects in human or mouse chondrocytes. This work aimed to verify the pathophysiological relevance of mCRP in the etiology and/or progression of osteoarthritis (OA). We investigated the effects of mCRP in cultured human primary chondrocytes and in the chondrogenic ATDC5 mouse cell line. We determined mRNA and protein levels of relevant factors involved in inflammatory responses and the modulation of nitric oxide synthase type II (NOS2), an early inflammatory molecular target. We demonstrate, for the first time, that monomeric C reactive protein increases NOS2, COX2, MMP13, VCAM1, IL-6, IL-8, and LCN2 expression in human and murine chondrocytes. We also demonstrated that NF-kB is a key factor in the intracellular signaling of mCRP-driven induction of pro-inflammatory and catabolic mediators in chondrocytes. We concluded that mCRP exerts a sustained catabolic effect on human and murine chondrocytes, increasing the expression of inflammatory mediators and proteolytic enzymes, which can promote extracellular matrix (ECM) breakdown in healthy and OA cartilage. In addition, our results implicate the NF-kB signaling pathway in catabolic effects mediated by mCRP.
Similar content being viewed by others
Introduction
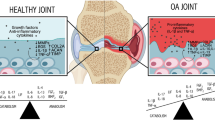
C-reactive protein (CRP) is an acute-phase reactant and a member of the pentraxin family [1]. It circulates prevalently in the blood as a pentamer of identical monomeric subunits. For many decades, CRP levels have been used as a circulating biomarker to monitor the inflammatory status in infections and other multiple diseases, including rheumatic diseases such as rheumatoid arthritis (RA) and osteoarthritis (OA) [2]. CRP has at least two naturally occurring and conformationally distinct isoforms described as pentameric CRP (pCRP) and monomeric CRP (mCRP) [3]. Defining the pathophysiological role of CRP as a regulator of inflammatory processes remains obscure and poorly described; elaborating on its role now requires careful attention to which CRP conformation contributes to any described response. OA is a degenerative disease mainly characterized by articular cartilage degradation. A combination of multiple factors, including genetic, mechanical, inflammatory, and metabolic, is likely to be involved in both pathogenesis and progression of the disease [4], but most of them still remain elusive.
CRP levels have been reported to be significantly elevated in patients with OA compared to healthy controls, and they were observed to be correlated with radiographic parameters as well as clinical severity [5,6,7]. Although some studies showed no association between CRP levels and radiographic OA, a correlation with pain and decreased physical function has been found [8]. Of interest, most studies in OA patients (2, 5–7) only quantified CRP levels in the high sensitivity range (i.e. hsCRP) describing values below 10 μg/ml. According to several studies [9,10,11,12,13,14,15] and to the guidance of US-FDA [16], the clinical significance of such values is undefined.
A recent paper by Kozijn et al. [17] demonstrated that CRP aggravates OA development in mice on a high-fat diet, although the mechanism of action for CRP involvement is not clarified yet. A hypothetical mechanism through which CRP could be involved in local cartilage disarrangements in OA might be via the pathogenic monomeric subunit of CRP (mCRP), originated by phospholipase-A2 (PLA2) enzymatic action in an inflammatory environment [18]. Increased PLA2 activity has been observed in synovial fluid from OA patients and in animal models of OA [19, 20]. However, it is also hypothetically possible that pCRP increases locally where it works to dampen the acute inflammatory response leading to prolonged, chronic tissue damage associated with arthritis. Whereas the phosphatidyl choline (PC) groups on PLA2 binding to the PC face will make the pCRP cholesterol binding ligand accessible so that pCRP can bind to a membrane, and, consequently, it is juxtaposed to a lipid zone that will supply the biochemical energy to relax pCRP into pCRP* orientation then dissociates to mCRP [21].
To date, the role of mCRP in cartilage pathophysiology, as well as its relevance in OA etiology and progression, has not been investigated. Therefore, in this study, we evaluated the functional role of mCRP as a modulator of the inflammatory response in chondrocytes. We evaluated the modulation of nitric oxide synthase type II (NOS2), an early inflammatory molecular mediator, as well as other relevant factors involved in the inflammatory response in human primary and immortalized mouse chondrocytes upon mCRP stimulation.
Materials and methods
Reagents
Fetal bovine serum (FBS), human transferrin, sodium selenite, lipopolysaccharide (LPS), mouse recombinant interleukin (IL)1β, and nuclear factor kappa B (NF-kB) inhibitor pyrrolidine dithiocarbamate (PDTC) were obtained from Sigma-Aldrich (St Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s F12 medium, trypsin-ethylenediaminetetraacetic acid, l-glutamine, and penicillin-streptomycin mixture were obtained from Lonza Group (Basel, Switzerland). Pronase and collagenase P were obtained from Roche Applied Science (Penzberg, Germany).
Recombinant mCRP produced in Escherichia coli had both cysteines replaced with alanine residues and was solubilized by an acylation technique (C-rmCRP) in 25 mM NaPBS (pH7.4), purified and tested in several bioassays to confirm its equivalence to biological mCRP from the pentameric form and free of contaminating endotoxin. The final endotoxin level of all protein solutions was below the detection limit 0.06 EU/ml of the assay [22].
Cell culture conditions and treatments
The murine chondrogenic cell line ATDC5 (RIKEN Cell Bank, Tsukuba, Japan) was cultured in DMEM/Ham’s F12 supplemented with 5% FBS, 10 μg/ml human transferrin, 3 × 10 M sodium selenite, 4 mM l-glutamine, 50 units/ml penicillin and 50 µg/ml streptomycin. Articular cartilage samples were obtained from knee and hip joints of patients undergoing total joint replacement, under written patient consent, and permission from the local ethics committee, according to the declaration of Helsinki. Human primary chondrocytes were isolated and cultured as previously described [23].
Chondrocytes were seeded in six-well plates (2.5 × 105 cells/well) and treated with 10, 25, or 50 µg/ml mCRP, 100 ng/ml LPS or 0.1 ng/ml IL-1β for 24 h, after serum starvation of human primary chondrocytes or ATDC5 cell line for 4 h or overnight, respectively. All the concentrations were selected based on previous studies and in the absence of chondrocyte cell toxicity. All treatments lasted 24 h, after 4 h of starvation for FBS in human primary chondrocytes and overnight starvation in ATDC5 cell line.
Nitrite assay
Nitrite accumulation was measured in the culture medium by the Griess reaction, as previously described [24,25,26]. Cell culture supernatant was incubated at room temperature with Griess reagent (equal volumes of 1% (w/v) sulfanilamide in 5% (v/v) phosphoric acid and 0.1% (w/v) naphtylethylenediamine-HCl), and the absorbance was measured at 550 nm in a microplate reader (Titertek Multiscan; Labsystems, Helsinki, Finland). Fresh culture medium was used as a blank, and nitrite concentration (μM) was calculated from a sodium nitrite standard curve.
RNA isolation and real-time reverse transcription-polymerase chain reaction
Total RNA was isolated from cell culture with NZYol (NZYTech, Lisbon, Portugal) and E.Z.N.A.® Total RNA Kit I (Omega Bio-tek, Inc., Norcross, GA, USA) according to the manufacturer’s instructions; and reverse-transcribed using NZY First-Strand cDNA Synthesis Kit (NZYTech, Lisbon, Portugal). Then, SYBR-green-based quantitative real-time PCR (RT-qPCR) was performed in Stratagene MX3005P thermal cycler as previously described [27] using a standard protocol with RT2 SYBR Green qPCR Mastermix and specific PCR primers (Qiagen, Hilden, Germany) (human GAPDH, 175 bp, PPH00150E, reference position 1287, Gen-Bank accession no. NM_002046.3; mouse GAPDH, 140 bp, PPM02946E, reference position 309, Gen-Bank accession no. NM_008084.2; human NOS2, 132 bp, PPH00173E, reference position 3962, Gen-Bank accession no. NM_000625.4; mouse NOS2, 122 bp, PPM02928B, reference position 2728-2748, Gen-Bank accession no. NM_010927.3; human MMP13, 61 bp, PPH00121B, reference position 1380, Gen-Bank accession no. NM_002427.3; mouse MMP13, 88 bp, PPM03675A, reference position 1145, Gen-Bank accession no. NM_008607; human VCAM1, 141 bp, PPH00623E, reference position 2980, Gen-Bank accession no. NM_001078; mouse VCAM1, 78 bp, PPM03208C, reference position 2307, Gen-Bank accession no. NM_011693; human LCN2, 87 bp, PPH00446E, reference position 626-644, Gen-Bank accession no. NM_005564.3; mouse LCN2, 81 bp, PPM03770A, reference position 364-383, Gen-Bank accession no. NM_008491.1; human IL6, 98 bp, PPH00560C, reference position 816, Gen-Bank accession no. NM_000600.3; mouse IL6, 178 bp, PPM03015A, reference position 120, Gen-Bank accession no. NM_031168; human IL8, 126 bp, PPH00568A, reference position 326, Gen-Bank accession no. NM_000584.3; human PTGS2, 63 bp, PPH01136F, reference position 1502, Gen-Bank accession no. NM_000963.2) No-template controls were included to eliminate any nonspecific amplification and melting curves were generated to ensure a single gene-specific peak. Gene expression changes were determined by the comparative (ΔΔCt) method in MxPro qPCR Software version 4.10 (Stratagene, La Jolla, CA, USA), and expressed as relative fold changes compared to control and normalized to GAPDH housekeeping gene.
Protein extraction and western blot analysis
After treatments, total cell lysates were obtained using lysis buffer (10 mM Tris–HCl, pH 7.5, 5 mM EDTA, 150 mM NaCl, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 0.5% Triton X-100, 1 mM PMSF), freshly supplemented with protease inhibitor cocktail (Thermo Fisher Scientific, Waltham, MA, USA), and then centrifuged at 14,000 g for 20 min. SDS-PAGE and blotting procedures were performed as previously described [28]. Immunoblots were incubated with the specific antibody against (NOS-2 or COX-2 (Cell Signaling, Danvers, MA, USA), IL-6 or IL-8 (Santa Cruz Biotechnology, Dallas, TX, USA), MMP13 (Abcam, Cambridge, UK), mouse LCN2, or human LCN2 (R&D Systems, Minneapolis, MN, USA)).
The immune complexes were detected using anti-rabbit (GE Healthcare, Chicago, IL, USA), anti-mouse (both from GE Healthcare, Chicago, IL, USA) or anti-goat (Santa Cruz Biotechnology, Dallas, TX, USA) horseradish-peroxidase-labeled secondary antibodies and Immobilon ECL. To verify equivalent protein loading, the membranes were incubated with anti-GAPDH antibody (Sigma-Aldrich).
The signals generated were detected in ChemiDoc MP Imaging System (Bio-Rad Laboratories, Inc.) and analyzed with Image Lab 6.0.1 Software (Bio-Rad Laboratories, Inc., Hercules CA, USA).
Statistical analysis
Data are reported as mean ± standard error of the mean (SEM) of at least three independent experiments. Statistical tests were applied using GraphPad Prism 6 software (GraphPad Software, La Jolla, CA, USA). Assuming a normal distribution, one-way ANOVA followed by Fisher´s LSD test was used. As we performed few planned comparisons, p values are not corrected for multiple comparisons, they apply individually to each value reported and not to the entire family of comparisons. P values less than 0.05 were considered significant.
Results
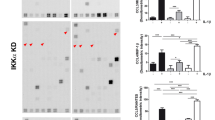
As shown in Fig. 1A, mCRP at both 10 and 50 μg/ml induces a significant accumulation of nitrite levels in the supernatant of cultured primary human chondrocytes from OA patients. Accordingly, mCRP increases NOS-2 mRNA expression and protein levels, as confirmed RT-qPCR and western blot analysis’ densitometry, respectively (1B). In a similar manner, mCRP also increased both mRNA and protein expression of COX-2 (C), MMP13 (D), VCAM1(E), IL-6 (F), IL-8 (G) and LCN2 (H).
Human primary OA chondrocytes in culture were challenged with 10, 25, or 50 µg/ml mCRP, or with 100 ng/ml LPS as positive control for 24 h, after 4 h serum starvation. A Nitric oxide production was evaluated by determining nitrite concentration (µM) in culture medium after 24 h treatment with 10, or 50 µg/ml mCRP, or with 100 ng/ml LPS as positive control using Greiss reaction. B–H (left panels) mRNA expression was determined by RT-qPCR. mRNA levels are presented as fold change relative to control (C−). B–H (middle and right panels) Protein expression was measured by western blot. Data from densitometric analyses normalized to GAPDH are shown as relative-to-control (C−) values. Data expressed as mean ± SEM of at least three independent experiments. Comparisons referred to control (C−): *P < 0.05, **P < 0.01, ***P < 0.001. mCRP monomeric C-reactive protein, LPS lipopolysaccharide, NOS2 nitric oxide synthase 2, PTGS2 prostaglandin-endoperoxide synthase 2, COX2 cyclooxygenase 2, MMP13 matrix metalloproteinase 13, VCAM1 vascular cell adhesion molecule 1, IL6 interleukin 6, IL8 interleukin 8, LCN2 lipocalin 2, GAPDH glyceraldehyde 3-phosphate dehydrogenase.
The induction of pro-inflammatory mediators by mCRP is also evident in human primary chondrocytes from healthy patients, suggesting that mCRP can modify the cell status by promoting the development of a pro-inflammatory environment in the cartilage (Fig. 2 from A to G). In all the experiments with human cells, LPS was used as positive control of inflammatory induction. Of note, the chondrocyte response to 50 µg/ml mCRP was comparable to that of 100 ng/ml LPS for the analyzed parameters.
Human primary healthy chondrocytes in culture were challenged with 10 or 50 µg/ml mCRP, or with 100 ng/ml LPS as positive control for 24 h, after 4 h of serum starvation. A–G (left panels) mRNA expression was determined by RT-qPCR. mRNA levels are presented as fold change relative to control (C−). A–G (middle and right panels) Protein expression was measured by western blot. Data from densitometric analyses, normalized to GAPDH, are shown as relative-to-control (C−) values. Data expressed as mean ± SEM of at least three independent experiments. Comparisons referred to control (C−): *P < 0.05, **P < 0.01, ***P < 0.001. mCRP monomeric C-reactive protein, LPS lipopolysaccharide, NOS2 nitric oxide synthase 2, PTGS2 prostaglandin-endoperoxide synthase 2, MMP13 matrix metalloproteinase 13, VCAM1 vascular cell adhesion molecule 1, IL6 interleukin 6, IL8 interleukin 8, LCN2 lipocalin 2.
To further confirm our results, we tested mCRP activity in the ATDC5 murine chondrocyte cell line, a very useful tool for studying molecular and cellular mechanisms of cartilage inflammation in vitro [26]. As shown in Fig. 3, mCRP significantly induces nitrite accumulation in ATDC5 cells (3A), and highly augments NOS2 (B), MMP13 (C), VCAM1 (D), IL-6 (E) and LCN2 (F), either at mRNA or protein level, as previously observed in human primary chondrocytes.
Murine chondrocyte cells were challenged with 10, 25, or 50 µg/ml mCRP, or with 100 ng/ml LPS or 0.1 ng/ml IL-1b as positive controls for 24 h, after overnight serum starvation. A Nitric oxide production was evaluated by determining nitrite concentration (µM) in culture medium after 24 h treatment using Greiss reaction. Data expressed as mean ± SEM of at least six independent experiments. B–F (left panels) mRNA expression was determined by RT-qPCR. mRNA levels are presented as fold change relative to control (C−) Data expressed as mean ± SEM of at least three independent experiments. B–F (middle and right panels) Protein expression was measured by western blot. Data from densitometric analyses normalized to GAPDH are shown as relative-to-control (C−) values. Data expressed as mean ± SEM of at least three independent experiments. Comparisons referred to control (C−): *P < 0.05, **P < 0.01, ***P < 0.001. mCRP monomeric C-reactive protein, LPS lipopolysaccharide, IL-1b interleukin 1 beta, NOS2 nitric oxide synthase 2, MMP13 matrix metalloproteinase 13, VCAM1 vascular cell adhesion molecule 1, IL6 interleukin 6, LCN2 lipocalin 2, GAPDH glyceraldehyde 3-phosphate dehydrogenase.
To gain further insights into the action mechanisms responsible by mCRP-induced pro-inflammatory effects in chondrocytes, we analyzed the involvement of NF-kB signaling pathway. NF-κB is a transcription factor that has been widely demonstrated to be involved in cellular responses to different signals of stress, such as cytokines, free radicals, heavy metals, and bacterial or viral antigens. Over-expression or inappropriate activation of NF-κB is implicated in many pathological mechanisms of diseases, ranging from inflammation to cancer [29]. PDTC is a thiol compound, which has been considered an effective NF-kB inhibitor [30]. Thus, in order to investigate the potential protective effects of NF-κB inhibition on mCRP-induced pro-inflammatory mediators’ expression, PDTC was used in combination with mCRP in chondrocytes. As shown in Fig. 4 (panels A and B), PDTC significantly reduce both nitrite accumulation and NOS2 expression (at mRNA and protein levels) in mCRP-stimulated ATDC5 cells. In addition, PDTC also significantly reduced the expression of MMP13, VCAM1, IL-6, and LCN2 induced by 50 µg/ml mCRP (Fig. 4, panels C to F)
Cells starved for serum overnight were pre-treated for 1 h with PDTC 5 µM. After keeping the culture medium, were challenged with 50 µg/ml mCRP for 24 h. A Nitric oxide production was evaluated by determining nitrite concentration (µM) in culture medium after 24 h treatment using Griess reaction. Data expressed as mean ± SEM of six independent experiments. Comparisons mCRP 50 µg/mL vs. mCRP 50 µg/mL + PDTC 5 µM. B–F (left panels) mRNA expression was determined by RT-qPCR. mRNA levels are presented as fold change relative to control (C−). Data are expressed as mean ± SEM of at least five independent experiments. B–F (middle and right panels) Protein expression was measured by western blot. Data from densitometric analyses, normalized to GAPDH, are shown as relative-to-control (C−) values. Data expressed as mean ± SEM of at least three independent experiments. Comparisons mCRP 50 µg/mL vs. mCRP 50 µg/mL + PDTC 5 µM. *P < 0.05, **P < 0.01, ***P < 0.001. mCRP monomeric C-reactive protein, NF-kB nuclear factor kappa B, PDTC pyrrolidine dithiocarbamate, NOS2 nitric oxide synthase 2, MMP13 matrix metalloproteinase 13, VCAM1 vascular cell adhesion molecule 1, IL6 interleukin 6, LCN2 lipocalin 2.
Discussion
CRP is a well-established biomarker in a plethora of infectious and inflammatory diseases, including RA and OA. Furthermore, CRP levels are closely associated with disease severity and/or progression [31], however little is known about its pathophysiological role. It is still matter of debate whether CRP plays any specific role in the elementary pathological processes.
CRP is an acute-phase protein that circulates in the blood prevalently as an annular (ring-shaped) pentamer of five identical monomer subunits (pCRP), which has been evidenced as a mediator of inflammatory and immune responses in the context of local tissue injury [32, 33]. At bone level, CRP has been reported to have controversial biological effects on osteoclast differentiation. However, recently, it has been demonstrated that only mCRP originated from local pCRP in the joint compartment, may bind to RANKL, thus, neutralizing its activity and downregulating osteoclast differentiation [34].
The physiological and pathological roles of mCRP and its biological relevance in cartilage biology and in OA is practically unknown.
Several published data regarding the relationship between OA development and progression and the levels of CRP are contradictory since CRP levels are in the range of hsCRP (below 10 μg/ml) whose clinical significance is matter of debate. These contradictory results, together with the lack of knowledge about the pathophysiological role of the mCRP in cartilage, have overshadowed the association between CRP levels and OA pathology, making the functional involvement of CRP in OA pathogenesis and/or progression uncertain. Evidence suggests that mCRP exhibits different antigenic, biological, and electrophoretic properties compared to the pentameric form and it generally displays marked pro-inflammatory properties in several cell lineages, including vascular endothelial cells, macrophages, and neutrophils [35,36,37].
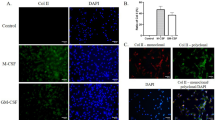
Although several lines of evidence have postulated a role for mCRP in cartilage, there is no experimental evidence of its activity in human or mouse chondrocytes. To address this key question, we tested the effect of mCRP in primary culture of human chondrocytes isolated from patients with hip and knee OA, or from healthy subjects. For the sake of completeness, the ATDC5 murine chondrogenic cell line was also used as another chondrocyte model for comparison. As far as we are aware, this is the first experimental article that provides evidence for the functional activity of mCRP in human and mouse chondrocytes. To gain further insights into the action of mCRP and to verify whether this stimulation can result in sustained pro-inflammatory effects, we evaluated the dose-dependent activity of mCRP in master inflammatory and catabolic players in cartilage degradation. Nitric oxide synthase type II (iNOS) and COX-2 are classically involved in the amplification of inflammatory responses. NO is cytotoxic to chondrocytes, damages cartilage, and is involved in the upregulation of MMPs, among which MMP13 is one of the major mediators of ECM degradation. COX-2 induces prostaglandins that in turn increase the synthesis of other inflammatory mediators, including cytokines, to perpetuate cartilage destruction [38]. Thus, we have focused on the regulation of iNOS and NO production, as well as other six major pro-inflammatory mediators with different functions in cartilage destruction, namely COX-2, MMP13, VCAM1, IL-6, IL-8, and LCN2. All these mediators are produced by chondrocytes and are elevated in arthritic joints [4, 39,40,41,42] Our results agree with those obtained by other authors in other cell types. For instance, Wang and collaborators demonstrated that CRP increases IL-8 gene expression in HUVEC cells [43]. Of note in Wang’s report, the effect of CRP on gene expression was measured after a 24-h cell culture incubation of CRP with vascular endothelial cells. It has been established that incubating CRP in cell culture or with liposomes for just 30 min, and notably within the first 24 h, can cause pCRP to change conformation in mCRP isomeric forms [30, 44,45,46,47]. As Wang’s report appeared prior to widespread awareness of distinct CRP structural isoforms with distinctive bioactivities, and in reflecting on the results on direct comparison of mCRP-specific bioactivities presented herein, Wang’s results may more precisely reflect on mCRP effects rather than pCRP.
Our present findings bring out important evidence regarding the modulation of articular phenotype and functions exerted by mCRP. First, exposure of chondrocytes to mCRP-induced multiple pro-inflammatory genes suggesting that its effects are not limited to a specific molecular target, rather mCRP has a broader spectrum of action. The second important point is that the effect of mCRP is persistent and sustained, regardless of the pro-inflammatory environment. Actually, mCRP exerted its action on human primary chondrocytes coming not only from OA patients, but also from healthy subjects. This latter finding suggests that elevated locally produced levels of mCRP may trigger sustained multigenic inflammatory responses also in normal tissues not previously exposed to a pro-inflammatory micro-environment, as in the case of OA chondrocytes. Of note, it was recently described that mCRP directly binds to fibronectin without depending on calcium presence and pH (acidic in the micro-environment of inflamed tissue, which promotes dissociation into the monomeric form). It was also shown that mCRP deposited in the ECM may regulate the recruitment and adhesion of monocytes thus triggering the inflammatory process [48]. This could help to understand the similar response to mCRP in both healthy and OA chondrocytes. The third important point is that the effects of mCRP in chondrocytes are likely to be mediated by NF-kB signaling pathway. In particular, our results demonstrate that pyrrolidine dithiocarbamate (a well-known pharmacological inhibitor of NF-kB) partially abrogates the expression of all pro-inflammatory genes induced by mCRP. Accordingly, in osteoclasts, mCRP has revealed to act primarily through NF-kB and phospholipase C pathways [49]. Furthermore, in endothelial cells, p38 MAPK was implicated in the mCRP-induced cytokine promotion [34, 50]. Although further experiments are needed due to the versatility of mCRP in activating different signaling pathways, we describe here, for the first time, the involvement of NF-kB in chondrocytes upon mCRP stimulation.
In conclusion, our present findings provide, for the first time, a molecular basis for the sustained action of mCRP in human and murine chondrocytes. Taken together, these results suggest that mCRP exerts a sustained catabolic effect by increasing the expression of inflammatory mediators and proteolytic enzymes, such as MMP13, and is thus able to promote cartilage breakdown and to trigger inflammatory responses in healthy and OA cartilage. Altogether, these molecules can cooperate resulting in the enhancement and perpetuation of the ECM-degrading processes at cartilage level. Finally, our results indicate that the route triggered by the release of NF-kB is involved. Despite these findings, further studies on the signaling elicited by mCRP are needed to completely assess its role in cartilage degradation and the pathogenesis and progression of OA.
References
Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754.
Hanada M, Takahashi M, Furuhashi H, Koyama H, Matsuyama Y. Elevated erythrocyte sedimentation rate and high-sensitivity C-reactive protein in osteoarthritis of the knee: relationship with clinical findings and radiographic severity. Ann Clin Biochem. 2016;53:548–53.
Schwedler SB, Filep JG, Galle J, Wanner C, Potempa LA. C-reactive protein: a family of proteins to regulate cardiovascular function. Am J Kidney Dis. 2006;47:212–22.
Francisco V, et al. Biomechanics, obesity, and osteoarthritis. The role of adipokines: When the levee breaks. J Orthop Res. 2018;36:594–604.
Sanchez-Ramirez DC et al. Elevated C-reactive protein is associated with lower increase in knee muscle strength in patients with knee osteoarthritis: A 2-year follow-up study in the Amsterdam Osteoarthritis (AMS-OA) cohort. Arthritis Res. Ther. 2014;16:R123.
Lee YC, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res. 2011;63:320–7.
Punzi L, et al. Value of C reactive protein in the assessment of erosive osteoarthritis of the hand. Ann Rheum Dis. 2005;64:955–7.
Jin X, et al. Circulating C reactive protein in osteoarthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:703–10.
Lowe GDO, Pepys MB. C-reactive protein and cardiovascular disease: weighing the evidence. Curr Atheroscl Rep. 2006;8:421–8.
Lloyd-Jones DM, Liu K, Tian L, Greenland P. Narrative review: assessment of C-reactive protein in risk prediction for cardiovascular disease. Ann Intern Med. 2006;145:35–42.
Sattar N, Hingorani AD. C-reactive protein and prognosis in diabetes: getting to the heart of the matter. Diabetes. 2009;58:798–9.
Elliott P, et al. Genetic loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302:37–48.
Yousuf O, et al. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62:397–408.
Chang YH, Hwu DW, Kao WP, Lee YJ. Benefits of rosuvastatin in cardiovascular protection remain unclear after HOPE-3. Rev Diabet Stud. 2016;13:212–4.
Antonelli M, Kushner I. It’s time to redefine inflammation. FASEB J. 2017;31:1787–91.
Review Criteria for Assessment of C Reactive Protein (CRP), High Sensitivity C-Reactive Protein (hsCRP) and Cardiac C-Reactive Protein (cCRP) Assays - Guidance for Industry and FDA Staff | FDA. Accessed 5 Oct 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/review-criteria-assessment-c-reactive-protein-crp-high-sensitivity-c-reactive-protein-hscrp-and.
Kozijn AE, et al. Human C-reactive protein aggravates osteoarthritis development in mice on a high-fat diet. Osteoarthr Cartil. 2019;27:118–28.
Thiele JR, et al. Dissociation of pentameric to monomeric C-reactive protein localizes and aggravates inflammation: In vivo proof of a powerful proinflammatory mechanism and a new anti-inflammatory strategy. Circulation. 2014;130:35–50.
Kortekangas P, Aro HT, Nevalainen TJ. Group II phospholipase a2 in synovial fluid and serum in acute arthritis. Scand J Rheumatol. 1994;23:68–72.
Panula HE, et al. Elevated levels of synovial fluid PLA2, stromelysin (MMP-3) and TIMP in early osteoarthrosis after tibial valgus osteotomy in young beagle dogs. Acta Orthop Scand. 1998;69:152–8.
Rajab IM, et al. C-reactive protein in gallbladder diseases: diagnostic and therapeutic insights. Biophys Rep. 2020;6:49–67.
Potempa LA, Yao Z-Y, Ji S-R, Filep JG, Wu Y. Solubilization and purification of recombinant modified C-reactive protein from inclusion bodies using reversible anhydride modification. Biophys Rep. 2015;1:18–33.
Otero M, et al. Human chondrocyte cultures as models of cartilage-specific gene regulation. Methods Mol. Biol. 2012;806:301–36.
Gõmez R, et al. Nitric oxide boosts TLR-4 mediated lipocalin 2 expression in chondrocytes. J Orthop Res. 2013;31:1046–52.
Otero M, et al. Phosphatidylinositol 3-kinase, MEK-1 and p38 mediate leptin/interferon-gamma synergistic NOS type II induction in chondrocytes. Life Sci. 2007;81:1452–60.
Santoro A, et al. Choosing the right chondrocyte cell line: focus on nitric oxide. J. Orthop. Res. 2015;33:1784–8.
Scotece M, et al. Oleocanthal inhibits catabolic and inflammatory mediators in LPS-activated human primary osteoarthritis (OA) chondrocytes through MAPKs/NF-κB pathways. Cell Physiol Biochem. 2018;49:2414–26.
Lago R, et al. A new player in cartilage homeostasis: adiponectin induces nitric oxide synthase type II and pro-inflammatory cytokines in chondrocytes. Osteoarthr Cartil. 2008;16:1101–9.
Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J Clin Investig. 2001;107:135–42.
Satriano J, Schlondorff D. Activation and attenuation of transcription factor NF-kB in mouse glomerular mesangial cells in response to tumor necrosis factor-α, immunoglobulin G, and adenosine 3’:5’-cyclic monophosphate. Evidence for involvement of reactive oxygen species. J Clin Investig. 1994;94:1629–36.
Wu Y, Potempa LA, El Kebir D, Filep JG. C-reactive protein and inflammation: conformational changes affect function. Biol Chem. 2015;396:1181–97.
Thiele JR, et al. Targeting C-reactive protein in inflammatory disease by preventing conformational changes. Mediators Inflamm. 2015;2015:9.
Khreiss T, et al. Loss of pentameric symmetry of C-reactive protein is associated with delayed apoptosis of human neutrophils. J Biol Chem. 2002;277:40775–81.
Jia Z-K, Li H-Y, Liang Y-L, Potempa LA, Ji S-R, Wu Y. Monomeric C-reactive protein binds and neutralizes receptor activator of NF-κB ligand-induced osteoclast differentiation. Front Immunol. 2018;9:234.
Slevin M, Krupinski J. A role for monomeric c-reactive protein in regulation of angiogenesis, endothelial cell inflammation and thrombus formation in cardiovascular/cerebrovascular disease? Histol Histopathol. 2009;24:1473–8.
El Kebir D, et al. C-reactive protein-derived peptide 201-206 inhibits neutrophil adhesion to endothelial cells and platelets through CD32. J Leukoc Biol. 2011;90:1167–75.
Ji S-R, et al. Monomeric C-reactive protein activates endothelial cells via interaction with lipid raft microdomains. FASEB J. 2009;23:1806–16.
Sun HB. Mechanical loading, cartilage degradation, and arthritis. Ann N. Y. Acad Sci. 2010;1211:37–50.
Francisco V, et al. Adipokines: Linking metabolic syndrome, the immune system, and arthritic diseases. Biochem Pharmacol. 2019;165:196–206.
Abella V, et al. Adipokines, metabolic syndrome and rheumatic diseases. J Immunol Res. 2014;2014:343746.
Khreiss T, József L, Potempa LA, Filep JG. Conformational rearrangement in C-reactive protein is required for proinflammatory actions on human endothelial cells. Circulation. 2004;109:2016–22.
Khreiss T, József L, Potempa LA, Filep JG. Loss of pentameric symmetry in C-reactive protein induces interleukin-8 secretion through peroxynitrite signaling in human neutrophils. Circ. Res. 2005;97:690–7.
Wang Q, et al. Effect of C-reactive protein on gene expression in vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2005;288:H1539–45.
De Lorgeril M, et al. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med. 2010;170:1032–6.
Ji SR, Wu Y, Potempa LA, Liang YH, Zhao J. Effect of modified C-reactive protein on complement activation: a possible complement regulatory role of modified or monomeric C-reactive protein in atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2006;26:934–41.
Ji S, et al. Cell membranes and liposomes dissociate C-reactive protein (CRP) to form a new, biologically active structural intermediate: mCRP m. FASEB J. 2007;21:284–94.
Ji SR, Wu Y, Potempa LA, Qiu Q, Zhao J. Interactions of C-reactive protein with low-density lipoproteins: Implications for an active role of modified C-reactive protein in atherosclerosis. Int J Biochem Cell Biol. 2006;38:648–61.
Ullah N, et al. Monomeric C-reactive protein regulates fibronectin mediated monocyte adhesion. Mol Immunol. 2020;117:122–30.
Boras E, et al. Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine. 2014;69:165–79.
Boras E, Slevin M, Gilmore W, Potempa LA, Matou-Nasri S. Common angiogenic signaling pathways induced by monomeric C-reactive protein and FGF-2 through MAPK and PI3K. Eur J Exp Biol. 2017;7:18.
Acknowledgements
OG and FL are Staff Personnel (I3SNS stable Researcher) of Xunta de Galicia (Servizo Galego de Saude, SERGAS) through a research-staff contract (ISCIII/SERGAS). VF is currently a “Sara Borrell” Researcher funded by ISCIII and FEDER (CD16/00111). RG and JC are “Miguel Servet” Researchers funded by Instituto de Salud Carlos III (ISCIII) and FEDER. CRF is a pre-doctoral research scholar funded by ISCIII and FEDER (Exp.18/00188). OG, MAGG, and RG are members of RETICS Programme, RD16/0012/0014 (RIER: Red de Investigación en Inflamación y Enfermedades Reumáticas) via Instituto de Salud Carlos III (ISCIII) and FEDER. FL is a member of CIBERCV (Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares). The work of OG and JP (PI17/00409), RG (PI16/01870) and FL (PI18/00821 and CB16/11/00226) was funded by Instituto de Salud Carlos III and FEDER. OG is a beneficiary of a project funded by Research Executive Agency of the European Union in the framework of MSCA-RISE Action of the H2020 Programme (Project number 734899). OG is beneficiary of a grant funded by Xunta de Galicia, Consellería de Educación, Universidade e Formación Profesional and Consellería de Economía, Emprego e Industria (GAIN), GPC IN607B2019/10.
Funding
The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
CRF, MG, and VF participated in acquisition of data, analysis, and interpretation of data and critical revision of the manuscript. AM, RG, JC, FL, JP, MAGG, and AM participated in the acquisition of data and samples, drafting of the manuscript, and statistical analysis. IMR participated in C-rmCRP preparation and editing the manuscript. LAP participated in the analysis and interpretation of data and drafting/editing the manuscript. OG participated in the conception and design of the study, in the analysis and interpretation of data, critical revision of the manuscript, and scientific supervision of experiments.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ruiz-Fernández, C., Gonzalez-Rodríguez, M., Francisco, V. et al. Monomeric C reactive protein (mCRP) regulates inflammatory responses in human and mouse chondrocytes. Lab Invest 101, 1550–1560 (2021). https://doi.org/10.1038/s41374-021-00584-8
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41374-021-00584-8
This article is cited by
-
Monomeric C-reactive Protein Exacerbates Neuronal Injury and Enhances Microglial Activation after Global Cerebral Ischemia in Mice
Molecular Neurobiology (2026)
-
From Homeostasis To Inflammation To Autoimmunity: The Potential Impact of CRP
Inflammation (2025)
-
Clinical and molecular links between chronic systemic inflammation and structural damage in axial spondyloarthritis
Arthritis Research & Therapy (2025)