Abstract
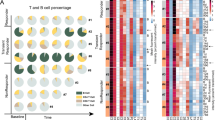
In Ph+ acute lymphoblastic leukemia, frontline dasatinib plus blinatumomab (dasa+blina) is associated with long-term survival rates of 75–80%. The phase III GIMEMA ALL2820 trial has explored ponatinib with blinatumomab (pona+blina). In the present study, the immune modulation induced by dasa+blina and pona+blina was investigated. Immune cells were analyzed at the end of induction (T0) and after 2, 4 and 5 blinatumomab cycles (T2, T4, T5). Among 153 patients (43 dasa+blina, 110 pona+blina), the dasa+blina combination induced a significantly greater lymphocyte increase at T4 and T5 compared to pona+blina. The Treg counts decreased only in the dasa+blina treated patients. NK and NK-T cells increased significantly in the dasa+blina group, at all timepoints. Complete molecular responders (CMR) after dasatinib induction had significantly higher lymphocytes, T and NK cells compared to non-CMR patients. Bone marrow analyses showed higher activation (CD25, CD69) and lower exhaustion (PD1, TIM3) markers on NK and NK-T cells in dasa+blina treated patients. Dasa+blina patients exhibited a significantly enhanced NK cell capacity compared to ponatinib treated patients. Patients remaining on dasatinib maintained elevated NK cells with a more mature phenotype, suggesting a durable effect. These results highlight the greater dasa+blina immune activation, supporting a potential synergistic effect of the drug combination.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Foà R, Chiaretti S. Philadelphia chromosome-positive acute lymphoblastic leukemia. N Engl J Med. 2022;386:2399–411.
Vignetti M, Fazi P, Cimino G, Martinelli G, Di Raimondo F, Ferrara F, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109:3676–8.
Foà R, Vitale A, Vignetti M, Meloni G, Guarini A, De Propris MS, et al. Dasatinib as first-line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118:6521–8.
Chiaretti S, Vitale A, Vignetti M, Piciocchi A, Fazi P, Elia L, et al. A sequential approach with imatinib, chemotherapy and transplant for adult Ph+ acute lymphoblastic leukemia: final results of the GIMEMA LAL 0904 study. Haematologica. 2016;101:1544–52.
Chiaretti S, Ansuinelli M, Vitale A, Elia L, Matarazzo M, Piciocchi A, et al. A multicenter total therapy strategy for de novo adult Philadelphia chromosome positive acute lymphoblastic leukemia patients: final results of the GIMEMA LAL1509 protocol. Haematologica. 2021;106:1828–38.
Martinelli G, Papayannidis C, Piciocchi A, Robustelli V, Soverini S, Terragna C, et al. INCB84344-201: Ponatinib and steroids in frontline therapy for unfit patients with Ph+ acute lymphoblastic leukemia. Blood Adv. 2022;6:1742–53.
Foà R, Bassan R, Vitale A, Elia L, Piciocchi A, Puzzolo MC, et al. Dasatinib-blinatumomab for Ph-positive acute lymphoblastic leukemia in adults. N Engl J Med. 2020;383:1613–23.
Foà R, Bassan R, Elia L, Piciocchi A, Soddu S, Messina M, et al. Long-term results of the dasatinib-blinatumomab protocol for adult philadelphia-positive ALL. J Clin Oncol. 2024;42:881–5.
Chiaretti S, Foà R. How I treat adult Ph+ ALL. Blood. 2025;145:11–9.
Foà R. Ph-positive acute lymphoblastic leukemia-25 years of progress. N Engl J Med. 2025;392:1941–52.
Puzzolo MC, Radice G, Peragine N, de Propris MS, Mariglia P, Vignetti M, et al. Host immune system modulation in Ph+ acute lymphoblastic leukemia patients treated with dasatinib and blinatumomab. Blood. 2021;138:2290–3.
Wan YY. Regulatory T cells: immune suppression and beyond. Cell Mol Immunol. 2010;7:204–10.
Dwarakanath BS, Farooque A, Gupta S. Targeting regulatory T cells for improving cancer therapy: Challenges and prospects. Cancer Rep. 2018;1:e21105.
Chiaretti S, Di Trani M, Skert C, Elia L, Almici G, Della Starza I, et al. First results of the phase III GIMEMA ALL2820 trial comparing ponatinib plus blinatumomab to imatinib and chemotherapy for newly diagnosed adult Ph+ acute lymphoblastic leukemia patients. Blood. 2025;146:439.
Elia L, Mancini M, Moleti L, Meloni G, Buffolino S, Krampera M, et al. A multiplex reverse transcriptase-polymerase chain reaction strategy for the diagnostic molecular screening of chimeric genes: a clinical evaluation on 170 patients with acute lymphoblastic leukemia. Haematologica. 2003;88:275–9.
van der Velden VH, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–11.
Pfeifer H, Cazzaniga G, van der Velden VHJ, Cayuela JM, Schäfer B, Spinelli O, et al. Standardisation and consensus guidelines for minimal residual disease assessment in Philadelphia-positive acute lymphoblastic leukemia (Ph + ALL) by real-time quantitative reverse transcriptase PCR of e1a2 BCR-ABL1. Leukemia. 2019;33:1910–22.
Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40.
Kantarjian H, Short NJ, Haddad FG, Jain N, Huang X, Montalban-Bravo G, et al. Results of the simultaneous combination of ponatinib and blinatumomab in Philadelphia chromosome-positive ALL. J Clin Oncol. 2024;42:4246–51.
Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre P, Paquette R, Chuah C, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369:1783–96.
Duell J, Dittrich M, Bedke T, Mueller T, Eisele F, Rosenwald A, et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia. 2017;31:2181–90.
Mustjoki S, Ekblom M, Arstila TP, Dybedal I, Epling-Burnette PK, Guilhot F, et al. Clonal expansion of T/NK-cells during tyrosine kinase inhibitor dasatinib therapy. Leukemia. 2009;23:1398–405.
Schiffer CA, Cortes JE, Hochhaus A, Saglio G, le Coutre P, Porkka K, et al. Lymphocytosis after treatment with dasatinib in chronic myeloid leukemia: Effects on response and toxicity. Cancer. 2016;122:1398–407.
Lee SJ, Jung CW, Kim DY, Lee KH, Sohn SK, Kwak JY, et al. Retrospective multicenter study on the development of peripheral lymphocytosis following second-line dasatinib therapy for chronic myeloid leukemia. Am J Hematol. 2011;86:346–50.
Najima Y, Yoshida C, Iriyama N, Fujisawa S, Wakita H, Chiba S, et al. Regulatory T cell inhibition by dasatinib is associated with natural killer cell differentiation and a favorable molecular response-The final results of the D-first study. Leuk Res. 2018;66:66–72.
Cao K, Wang X, Wang H, Xu C, Ma A, Zhang Y, et al. Phenotypic and functional exhaustion of circulating CD3+ CD56+ NKT-like cells in colorectal cancer patients. FASEB J. 2024;38:e23525.
Zeng X, Yao D, Liu L, Zhang Y, Lai J, Zhong J, et al. Terminal differentiation of bone marrow NK cells and increased circulation of TIGIT+ NK cells may be related to poor outcome in acute myeloid leukemia. Asia Pac J Clin Oncol. 2022;18:456–64.
Lou C, Wu K, Shi J, Dai Z, Xu Q. N-cadherin protects oral cancer cells from NK cell killing in the circulation by inducing NK cell functional exhaustion via the KLRG1 receptor. J Immunother Cancer. 2022;10:e005061.
Zhang Y, Chen S, Tang X, Peng Y, Jiang T, Zhang X, et al. The role of KLRG1: a novel biomarker and new therapeutic target. Cell Commun Signal. 2024;22:337.
Xu C, Cao K, Ma A, Zheng M, Xu Y, Tang L. KLRG1 expression induces functional exhaustion of NK cells in colorectal cancer patients. Cancer Immunol Immunother. 2025;74:203.
Golden-Mason L, McMahan RH, Strong M, Reisdorph R, Mahaffey S, Palmer BE, et al. Galectin-9 functionally impairs natural killer cells in humans and mice. J Virol. 2013;87:4835–45.
Venglar O, Bago JR, Motais B, Hajek R, Jelinek T. Natural killer cells in the malignant niche of multiple myeloma. Front Immunol. 2022;12:816499.
Yin M, Di G, Bian M. Dysfunction of natural killer cells mediated by PD-1 and Tim-3 pathway in anaplastic thyroid cancer. Int Immunopharmacol. 2018;64:333–9.
Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. CD28 costimulation: from mechanism to therapy. Immunity. 2016;44:973–88.
Yan Z, Wang C, Wu J, Wang J, Ma T. TIM-3 teams up with PD-1 in cancer immunotherapy: mechanisms and perspectives. Mol Biomed. 2025;6:27.
Ocadlikova D, Lussana F, Fracchiolla N, Bonifacio M, Santoro L, Delia M, et al. Blinatumomab differentially modulates peripheral blood and bone marrow immune cell repertoire: A Campus ALL study. Br J Haematol. 2023;203:637–50.
Zugmaier G, Gökbuget N, Klinger M, Viardot A, Stelljes M, Neumann S, et al. Long-term survival and T-cell kinetics in relapsed/refractory ALL patients who achieved MRD response after blinatumomab treatment. Blood. 2015;126:2578–84.
Gökbuget N, Dombret H, Bonifacio M, Reichle A, Graux C, Faul C, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131:1522–31.
Jabbour E, Zugmaier G, Agrawal V, Martínez-Sánchez P, Rifón Roca JJ, Cassaday RD, et al. Single agent subcutaneous blinatumomab for advanced acute lymphoblastic leukemia. Am J Hematol. 2024;99:586–95.
Dragani M, Ansuinelli M, Papayannidis C, Fracchiolla N, Cardinali V, Cedrone M, et al. Tyrosine kinase inhibitor discontinuation in nonallografted Philadelphia-positive acute lymphoblastic leukemia patients: a Campus ALL real-life study. Haematologica. 2025;110:1177–81.
Acknowledgements
The authors wish to thank Associazione Italiana per la Ricerca sul Cancro (AIRC) 5×1000, Special Program Metastases (21198), Milan (Italy) to R.F.
Author information
Authors and Affiliations
Contributions
M.A. and N.P. performed experiments, analyzed data and wrote the manuscript. M.S.D.P. and M.C.P. performed experiments and analyzed data. M.D.T. and L.E. performed experiments. C.S., R.C., S.S., A.R., A.T., M.C., D.V., V.C., A.M. provided clinical data and took care of the patients. S.C. designed clinical trials, provided clinical data, took care of patients and critically reviewed the manuscript. A.G. designed the research, analyzed the data, wrote and critically revised the manuscript; R.F. designed clinical trials and the research, analyzed the data, wrote and critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to disclose. The results of this study have been presented in part at the last ASH Congress: Ansuinelli M et al., Blood 2024;144 (Supplement 1):838.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41375_2025_2855_MOESM1_ESM.docx
Supplementary data for: IMMUNOMODULATORY EFFECT OF DASATINIB PLUS BLINATUMOMAB VERSUS PONATINIB PLUS BLINATUMOMAB IN NEWLY DIAGNOSED Ph+ ACUTE LYMPHOBLASTIC LEUKEMIA
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ansuinelli, M., Peragine, N., De Propris, M.S. et al. Immunomodulatory effect of dasatinib plus blinatumomab versus ponatinib plus blinatumomab in newly diagnosed Ph+ acute lymphoblastic leukemia. Leukemia (2026). https://doi.org/10.1038/s41375-025-02855-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41375-025-02855-5