Abstract
Amygdala hyperexcitability is a hallmark of stress-induced anxiety disorders. Stress-associated changes in both principal neurons and interneurons contribute to the increased excitability, but how exactly these mechanisms interact to regulate the function of behaviorally relevant circuits in the amygdala remains unclear. Here, we show that GluK1 subunit-containing kainate receptors in parvalbumin (PV) interneurons maintain high GABA release and control excitability of lateral amygdala (LA) principal neurons via tonic GABAB-receptor-mediated inhibition. Downregulation of GluK1 expression in PV interneurons after chronic restraint stress (CRS) releases the tonic inhibition and increases excitability of LA principal neurons. Stress-induced LA hyperexcitability was associated with increased glutamatergic transmission to central amygdala PKCδ-expressing neurons, implicated in fear generalization. Consistent with significance in anxiogenesis, absence of GluK1-GABAB regulation confers resilience against CRS-induced LA hyperexcitability and anxiety-like behavior. Our data reveal a unique novel mechanism involving an interplay between glutamatergic and GABAergic systems in the regulation of amygdala excitability in response to chronic stress.
Similar content being viewed by others
Introduction
Chronic stress produces lasting structural and functional changes in various areas of the brain, which contribute to the neuropathology of stress-related psychiatric diseases. Amygdala is one of the key structures implicated in the stress response in both humans and animal models. Stress hormones increase the discharge rates and firing synchrony of basolateral amygdala (BLA) neurons, which facilitates fear and aversive learning that is critical for animal survival [1]. During prolonged and severe stress, however, these mechanisms may become maladaptive and result in sustained hyperexcitability of BLA principal neurons [2]. Excessive activity of the BLA disturbs the regulation of downstream brain areas involved in emotional responses and is associated with various behavioral disorders characterized by unwarranted fear and anxiety [3,4,5,6,7].
The cellular mechanisms underpinning stress-induced amygdala hyperexcitability have been widely studied and shown to involve alterations in both the intrinsic properties of the principal neurons (PNs) and the surrounding network [7]. Chronic stress perturbs inhibitory control of PNs and is associated with reduced phasic and tonic GABAergic drive in the BLA [6]. Stress particularly affects the function of parvalbumin–expressing (PV) GABAergic interneurons, which mediate perisomatic inhibition of the PNs and tightly control their excitability and local network dynamics [5, 8, 9]. PV interneurons respond to stress in an age- and sex-specific manner [10] and their activity in BLA has been directly linked to anxiety-like behaviors in mice [11]. Yet, how exactly stress-dependent changes in PV interneurons contribute to the hyperexcitability of the PNs and how this affects the intra-amygdaloid circuit regulating fear-related behaviors remains poorly understood. For example, it is not known whether PV interneurons control PN excitability merely via fast inhibition or whether GABA release and spillover from these cells can also support tonic inhibition. Also, most studies on stress and GABAergic inhibition do not discriminate between the LA and BA nuclei of the BLA, despite the differences in their connectivity and functional roles [12, 13].
To get further insight into the mechanisms underlying aberrant behaviors induced by chronic stress, we have here focused on the trisynaptic circuit involving PV interneurons and principal neurons (PN) in the lateral amygdala (LA), as well as their GABAergic PKCδ−expressing target neurons in the centrolateral (CeL) amygdala. CeL PKCδ neurons are in a key position in controlling amygdala output and have been strongly implicated in regulation of anxiety and fear generalization [14,15,16]. We show that the excitability of LA PNs is regulated by tonic GABAB-mediated inhibition that is maintained by GABA release from PV interneurons, facilitated by kainate-type glutamate receptors (KARs). This mechanism is lost after chronic stress, due to downregulation of GluK1 KAR subunit expression specifically in PV interneurons. The increase in BLA excitability in the absence of tonic GluK1-GABAB-mediated inhibition was associated with a shift in the balance of excitatory drive towards PKCδ neurons in the CeL. Consistent with significance in fear generalization and anxiogenesis, ablation of GluK1 specifically in PV interneurons GluK1 expression conferred resilience against CRS-induced LA hyperexcitability and anxiety-like behavior.
Results
Loss of GABAB receptor-mediated tonic inhibition contributes to amygdala hyperexcitability after chronic stress
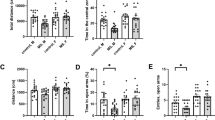
To study the effect of chronic stress on amygdala circuitry and anxiety-like behaviors, we subjected mice to chronic restraint stress protocol (CRS) involving 1 h immobilization in a ventilated tube during ten consecutive days. As expected, CRS was associated with significant loss of weight (Fig. 1A) as well as increase in the anxiety-like behavior in the open field test, where CRS-exposed mice spent significantly less time in the center area of the open field arena as compared to controls (Fig. 1B). In addition, CRS-exposed mice showed higher locomotor activity, indicated by the total distance traveled during the test (Fig. 1B). Yet, consistent with an anxiety-like phenotype, the ratio of distance traveled in the center field to the total distance in CRS group was lower as compared to controls (48 ± 3% and 62 ± 4%, respectively, p < 0.05, unpaired t-test).
A A scheme of the experimental protocol (top) and a graph illustrating body weight changes during the chronic restraint stress (CRS, n = 10, control n = 10; RM ANOVA F(1, 18) = 10.54, **p = 0.0045). B Results of the open field (OF) test. The graphs show the total time spent in the center area of the open field (OF), the center time in 5 min bins (control, n = 10, CRS, n = 10, t-test t = 3.232, df = 18, **p = 0.0046; RM ANOVA F(1, 18) = 10.45, **p = 0.0046) and total distance traveled during OF test (t-test, t = 4.546, df = 18, ***p = 0.0003). C Action potential (AP) firing rate of principal neurons (PN) in the lateral amygdala (LA) in response to depolarizing current steps, recorded from brain slices of control and CRS-exposed mice (control, n = 17 (8 mice), CRS, n = 19 (7 mice); RM ANOVA F (1, 29) = 4.877, *p = 0.035). The example traces illustrate the response to 240 pA step current. D Representative traces and pooled data illustrating the effect of CRS on sIPSC frequency and amplitude in LA PNs. Recordings were done using high-Cl containing electrode filling solution and in the presence of antagonists for AMPA and NMDA receptors (control, n = 12 (4 mice), CRS, n = 15 (4 mice), frequency: t-test, t = 1.381, df = 25, p = 0.1796; amplitude: t-test, t = 1.276, df = 25, p = 0.2136). The decay time distribution of sIPSCs for the same data is shown below (multiple t-test, Holm-Sidak, 1 ms: t = 3.070 df = 390.0, *p = 0.027; 2 ms : t = 3.250 df = 390.0, *p = 0.016). E Tonic GABAB receptor-mediated currents recorded from LA PNs in response to application of GABAB antagonist CGP55845 (10 μM), in control and CRS-exposed mice as well as in the presence of GDP-β-S in control mice. All recordings were done in the presence of 50 μM of D-AP5, 200 μM picrotoxin, and 50 μM GYKI 53655 to block NMDA, GABAA, and AMPA receptors, respectively. Pooled data on the maximal amplitude of the GABAB current under control conditions and in the presence of GDP-β-S (750 µM) in the electrode filling solution (control, n = 8 (3 mice), GDP-β-S n = 5 (3 mice); t-test, t = 4.599, df = 11, ***p = 0.0008). Amplitudes of the tonic GABAB current in control and CRS-exposed animals (control, n = 17 (7 mice), CRS, n = 14 (3 mice); t-test t = 3.786, df = 29, ***p = 0.0007). F Effect of GABAB antagonism on firing frequency of LA PNs in control and CRS-exposed mice, as well as in the presence of GDP-β−S in control mice. Action potential frequencies in response to depolarizing current steps were recorded from brain slices under control conditions (in ACSF) and in the presence of CGP55845 (5 µM) (control, n = 10 (4 mice), control+CGP55845, n = 10 (4 mice); RM ANOVA, F(2, 26) = 3.498, *p = 0.045; GDP-β−S n = 12 (3 mice), GDP-β−S+CGP55845 n = 10 (3 mice); RM ANOVA F(1,20) = 0.8588, p = 0.365; CRS, n = 17 (6 mice), CRS+CGP55845, n = 9 (3 mice); RM ANOVA, F(1, 24) = 1.824, p = 0.1894). Example traces show the response to 240 pA current step. All the data are presented as mean ± SEM.
After validating the CRS protocol, we went on to study the excitability of LA principal neurons (PNs) using whole-cell current-clamp recordings in acute brain slices from control and CRS-exposed mice. Consistent with previous reports [17, 18], the firing rate of the LA PN’s in response to depolarizing current steps was significantly higher in CRS-exposed mice as compared to controls (Fig. 1C). This effect was associated with lower amplitude of medium duration after-hyperpolarizing current (AHP) [17,18,19] and more depolarized resting membrane potential (control −61.44 ± 1.47; CRS −57.10 ± 1.32 mV, p = 0.03, t-test) (Supplementary Fig. 1A, B). No other differences in the properties of action potentials were detected between the groups (AP half-width, AP threshold, amplitude of the fast AHP current; Supplementary Fig. 1A).
Previously, it has been reported that CRS results in significant alterations in both glutamatergic and GABAergic synaptic inputs to the BLA principal neurons [7, 11, 20, 21]. However, we observed no significant differences between the control and CRS groups in the frequency or amplitude of spontaneous glutamatergic or GABAergic synaptic responses (sEPSCs and sIPSCs, respectively), that were recorded simultaneously under voltage clamp from LA PNs (Supplementary Fig. 1C). Since GABAergic input originates from distinct subtypes of interneurons that might be differentially regulated by CRS, we went on to record sIPSCs under modified conditions, ie. with high-chloride concentration in the electrode filling solution, to be able to distinguish fast (perisomatic) and slower (distal) synaptic events based on their kinetics. Analysis of the decay time distribution of the sIPSCs revealed that in the CRS group, the proportion of fast-decaying sIPSCs was lower as compared to controls, consistent with reduced perisomatic inhibition (Fig. 1D).
GABAergic activity can influence target cells also via extrasynaptic, tonic inhibition [22]. Chronic stress reduces tonic inhibition mediated by extrasynaptic GABAA-receptors in the BLA, which contributes to hyperexcitability of BLA PN’s [23, 24]. Recently, also GABAB-receptors have been implicated in tonic inhibition of the BLA principal neurons through activation of G-protein coupled inwardly rectifying potassium current [25, 26], yet their role in stress-induced amygdala hyperexcitability is not known. We observed that application of CGP55845, a selective GABAB-receptor antagonist, resulted in a significant shift in the holding current of the LA principal neurons, and this shift was fully blocked when G-protein coupled signaling was prevented by inclusion of the GDP-β−S in the recording electrode (Fig. 1E). This CGP55845-sensitive tonic GABAB current was significantly smaller in the CRS-exposed mice as compared to controls (Fig. 1E). Furthermore, current clamp recordings indicated that application of CGP55845 resulted in a significant increase in the firing rate of the LA PNs in response to depolarizing current steps in control slices, but not in slices from CRS-treated mice, nor in the presence of GDP-β−S in the recording electrode (Fig. 1F). Together, these data demonstrate that loss of tonic GABAB receptor-mediated inhibition contributes to the increased firing rate of LA PNs after chronic stress.
GABABR-dependent tonic inhibition of LA PNs is driven by GluK1 kainate receptors in PV interneurons
GluK1 subunit containing kainate receptors (KARs) have been previously shown to regulate both phasic and tonic GABAA receptor-mediated inhibition in the BLA [27,28,29], yet the subtypes of GABAergic interneurons responsible for this regulation are not known. Recently, we found that GluK1 KARs are endogenously active in PV interneurons in the LA and contribute to their high excitability [29]. To investigate the role of these KARs in tonic inhibition of LA PNs, we performed a set of pharmacological experiments in mice with floxed GluK1 gene (Grik1fl/fl, controls) and mice lacking GluK1 expression selectively in PV interneurons (PV-Cre::Grik1fl/fl).
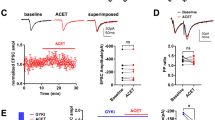
Tonic GABAA currents, recorded in response to application of bicuculline (25 µM) in LA PNs, were not significantly different between the genotypes (Fig. 2A). Interestingly, however, the CGP55845-sensitive tonic GABAB receptor-mediated current was smaller in mice lacking GluK1 expression selectively in PV interneurons as compared to controls (Fig. 2B). Furthermore, while in control mice the tonic GABAB current was substantially reduced in response to application of ACET (200 nM, Fig. 2B), a selective antagonist of GluK1 KARs [30, 31], ACET had no effect on CGP55845 sensitive currents in PV-Cre::Grik1fl/fl mice (p = 0.51; Fig. 2B). These differences were not due to the loss of postsynaptic GABAB receptors in the mutant mice, as the currents induced by the GABAB agonist SKF97541 in LA PNs were similar between the genotypes (Supplementary Fig. 1D).
A Tonic GABAA currents recorded as a change in the holding current in response to the application of 25 μM bicuculline in LA PNs, in slices from mice with floxed GluK1 gene (Grik1fl/fl) and mice lacking GluK1 expression selectively in PV interneurons (PV-Cre::Grik1fl/fl). All recordings were done at −90 mV holding potential in the presence of 50 μM of D-AP5 and 50 μM GYKI 53655 to block NMDA and AMPA receptors, respectively. The graph illustrates averaged data on the amplitude of the tonic GABAA current (Grik1fl/fl: n = 10 (4 mice); PV-Cre::Grik1fl/fl: n = 9 (3 mice); t-test, t = 1.681, df = 17, p = 0.11). B Tonic GABAB currents recorded from LA PNs in slices from mice with floxed GluK1 gene (Grik1fl/fl) and mice lacking GluK1 expression selectively in PV interneurons (PV-Cre::Grik1fl/fl), before and after ACET application (200 nM). All recordings were done at −50 mV holding potential in the presence of 50 μM of D-AP5, 200 μM picrotoxin, and 50 μM GYKI 53655 to block NMDA, GABAA, and AMPA receptors, respectively. The amplitude of the tonic current was measured as a change in the holding current in response to the application of 10 μM CGP55845. The graph illustrates averaged data on the amplitude of the tonic GABAB currents under various conditions (Grik1fl/fl: control, n = 17 (5 mice), ACET, n = 11 (4 mice); PV-Cre::Grik1fl/fl: control, n = 16 (4 mice), ACET, n = 7 (3 mice); Dunnett test, ***p < 0.001, **p = 0.0095). C Action potential firing rate of LA PNs in response to depolarizing current steps under control conditions and in the presence of ACET (200 nM). Recordings were done in brain slices from Grik1fl/fl and PV-Cre::Grik1fl/fl mice (Grik1fl/fl: control, n = 17 (5 mice), ACET, n = 17 (5 mice), RM ANOVA, F(1, 32) = 5.967, *p = 0.02; PV-Cre::Grik1fl/fl: control, n = 24 (8 mice), ACET, n = 22 RM ANOVA, F(1, 44) = 1.534, p = 0.22). D Action potential (AP) firing rate of LA PNs in response to depolarizing current steps under control conditions and in the presence of CGP55845 (5 µM), in PV-Cre::Grik1fl/fl mice (control, n = 15 (3 mice), CGP55845, n = 13 (3 mice), RM ANOVA, F(1, 44) = 0.1779, p = 0.6766). The example traces in C and D illustrate the response to the 240 pA current step. All the data is presented as mean ± SEM. See Supplementary Data 2 for the membrane properties of LA principal neurons in Grik1fl/fl and PV-Cre::Grik1fl/fl mice.
Consistent with GluK1 KARs contributing to tonic inhibition of LA PNs, GluK1 antagonism by ACET resulted in a significant increase in the firing rate of the LA PNs in response to depolarizing current steps in control mice (Fig. 2C). However, ACET had no effect when the same experiment was repeated in mutant mice lacking GluK1 expression in the PV interneurons (Fig. 2C). Furthermore, in contrast to controls (Fig. 1F), GABAB antagonism with CGP55845 had no effect on the excitability of LA PN’s in PV-Cre::Grik1fl/fl mice (Fig. 2D). In line with loss of tonic GABAB-mediated inhibition, the resting membrane potential of the LA PN’s in PV-Cre::Grik1fl/fl mice was slightly higher as compared to controls (−57.46 ± 1.8 mV vs −61.67 ± 0.97 mV, respectively, p = 0.047, unpaired t-test; Supplementary Data 2). However, we detected no differences between the genotypes in LA PN excitability, possibly due to developmental compensation (Fig. 2C; RM ANOVA for Grik1fl/fl vs PV-Cre::Grik1fl/fl, F (1, 36) = 0.6403, p = 0.429). Together, these data indicate that GluK1 KARs, located in the PV interneurons, are physiologically activated to regulate excitability of the LA PNs via GABAB receptor-mediated tonic inhibition.
GluK1 KARs facilitate GABA release in PV interneurons
KARs have been implicated in regulation of GABA release [32,33,34,35,36,37,38], yet no direct evidence on GluK1 KARs regulating release in PV interneurons exists. Therefore, we went on to investigate whether KARs regulate action potential-dependent and/or asynchronous GABA release from PV interneurons and thereby contribute to the ambient levels of GABA, mediating tonic inhibition. To this end, we activated PV interneurons in the BLA by using cell type-specific optogenetic stimulation and recorded pharmacologically isolated GABAergic responses from LA principal neurons (Fig. 3A). GluK1 KAR antagonism with ACET (200 nM) significantly reduced the amplitude of light-evoked IPSC and increased paired-pulse facilitation (Fig. 3B), consistent with presynaptic GluK1 KARs facilitating GABA release in PV interneurons in the LA.
A A scheme of the experimental protocol (left). AAV viral vectors encoding for Cre-dependent ChR2 were injected to BLA of PV-Cre mice for PV neuron-specific expression of ChR2. GABAergic responses were recorded from LA PNs in response to light stimulation. B Examples of IPSCs, evoked by paired-pulse stimulation with 470 nm blue light before and after 200 nM ACET application. Pooled data on the effect of ACET on the 1st IPSC amplitude and paired-pulse ratio (PPR) (n = 9 (5 mice), PPR, t = 4.567, df = 8, **p = 0.0018; IPSC, t = 3.246, df = 8, *p = 0.0118 paired t-test). C Asynchronous barrage of IPSCs recorded from LA PNs in response to 750 ms opto-stimulation of PV interneurons, before and after application of 200 nM of ACET. Pooled data illustrating the frequency of IPSCs, normalized to the level before opto-stimulation and analysed in 10 min bins under control conditions and in the presence of ACET (n = 8 (6 animals), RM ANOVA, F (1, 7) = 11.49, *p = 0.012). D The decay time distribution of sIPSCs at baseline, and during 10 s period after opto-stimulation of PV interneurons. Optogenetic activation of the PV interneurons increased the relative occurrence of sIPSCs with fast decay time (multiple t-test, Holm-Sidak; 1 ms : t = 3.486, df = 130.0, **p = 0.008). E The decay time distribution of sIPSCs at baseline and in the presence of ACET, during periods when opto-stimulation was not applied. In the presence of ACET, there was a lower percentage of 1 ms decay time sIPSC events (multiple t-test, Holm-Sidak, t = 3.566, df = 299.0, **p = 0.005466). All the data are presented as mean ± SEM.
Asynchronous GABA release from PV interneurons was evoked by prolonged light-induced depolarization, which induced a sustained increase in the frequency of sIPSCs in LA principal neurons (Fig. 3C). As shown previously [29], application of ACET resulted in a significant increase in the basal sIPSC frequency in LA PNs (baseline: 10.22 ± 1.366 Hz; ACET: 16.10 ± 3.003 Hz, paired t-test, t = 2.661, df = 7, *p = 0.0324; not shown), due to enhanced activity of somatostatin expressing interneurons upon release of PV neuron-mediated inhibition [29]. In the presence of ACET, however, the light-induced increase in sIPSC frequency, reflecting asynchronous GABA release from PV interneurons, was significantly smaller as compared to the control conditions (Fig. 3B, C). Analysis of the decay time distribution of the light-evoked IPSCs confirmed that activation of PV interneurons evokes IPSCs with fast decay (1 ms; Fig. 3D). By using the same analysis on spontaneous synaptic events, we confirmed that ACET reduced the percentage of fast-decaying sIPSCs (Fig. 3E), consistent with reduced PV interneuron-mediated perisomatic inhibition. Together, these data indicate that presynaptic KARs in PV interneurons are endogenously active and facilitate both action potential-dependent and asynchronous GABA release.
Chronic stress associates with loss of GluK1 expression and function in LA PV interneurons
Our data so far indicate that GluK1 KARs, facilitating GABA release from PV interneurons, maintain tonic GABAB-mediated inhibition of the LA PNs. In order to understand whether this mechanism is regulated by chronic stress, we went on to investigate whether CRS affects GluK1 expression in PV interneurons in the LA. Since the antibodies against GluK1 subunit of KARs have low specificity in brain sections, we performed triple in situ hybridization (ISH) using fluorescent probes against Grik1 (GluK1), Pvalb (parvalbumin) and Gad1 (GAD67, marker of the GABAergic neurons).
In control sections, Grik1 ISH signal was detected in most Gad1 positive GABAergic interneurons (Grik1+Gad1+/Gad1+, 94 ± 1%). Pvalb expressing neurons represented a subpopulation (23 ± 2%) of the Gad1 expressing neurons, which typically co-expressed Grik1 (Grik1+Pvalb+/Pvalb+, 96 ± 2%) (Fig. 4A).
A Representative images illustrating triple in situ hybridization staining for Grik1 (magenta), Gad1 (white), and Pvalb (green) in the LA in control and CRS-treated mice. The image with merged channels also shows DAPI staining (blue). Yellow arrows in the merged image point to cells co-expressing Grik1, Gad1 and Pvalb. B Bar charts summarizing the density of cells expressing Grik1 and Pvalb in the LA in control and CRS-treated mice. The Grik1 expression level is expressed as a percentage of DAPI stained nuclei, and Pvalb as a percentage of all Gad1 positive GABAergic cells (control, n = 20 sections (3 mice), CRS, n = 21 sections (3 mice), Grik1: t-test, t = 0.04723, df = 39, p = 0.9626; Pvalb: t = 0.05947, df = 42 p = 0.9529). The values represent mean ± SEM. C Bar charts summarizing the percentage of PV neurons (Gad1+Pvalb+) and other subtypes of GABAergic interneurons (Gad1+Pvalb-) co-expressing Grik1 mRNA in LA of control and CRS-exposed mice (Pvalb+: control, n = 14 section (3 mice), CRS, n = 15 (3 mice), Mann-Whitney U = 38.50, **p = 0.001; Gad1+Pvalb+: control, n = 13 section (3 mice), CRS, n = 15 (3 mice), GAD: t = 1.594, df = 26, p = 0.1230). The values represent mean ± SEM. D High-magnification images illustrating the expression of Grik1 mRNA (magenta) in individual Pvalb positive LA neurons (left), and in Pvalb negative, Gad1 expressing neurons (Pvalb, green, Gad1, white). The image with merged channels also shows DAPI staining (blue). Pooled data summarizing the average intensity of Grik1 mRNA staining (# dots) per cell, in PV neurons (Pvalb+) and in other subtypes (Gad1+Pvalb-) of GABAergic interneurons in LA of control and CRS animals (Pvalb+: control, n = 22 sections from 3 animals, CRS, n = 19 sections from 3 animals, Mann-Whitney test, U = 128, *p = 0.034; Pvalb-Gad1+: control, n = 14 sections from 3 animals, CRS, n = 15 sections from 3 animals, Mann-Whitney test, U = 100.5, p = 0.8554). Data are presented as median and quartile. Histogram demonstrating the distribution of Grik1 mRNA staining intensity in individual PV neurons in control and CRS mice (control, n = 60 cells, CRS, n = 46 cells, 3 animals in both groups, Kolmogorov-Smirnov test, D = 0.4841, ****p < 0.0001). E Current clamp recordings from PV interneurons in acute slices from control and CRS-exposed PV-TdTomato mice. Example traces illustrate the response of the PV neurons to depolarizing currents steps (50 pA and 200 pA), for control and CRS groups. Pooled data on the action potential frequencies in response to depolarizing current steps (control, n = 17 (7 mice), CRS, n = 18 (6 mice), RM ANOVA F(1, 32) = 4.370, *p = 0.0446). F Example traces illustrate the response of the PV neurons before and after ACET application (50 pA current step), in control and CRS-exposed PV-TdTomato mice. Pooled data on the action potential frequencies in response to depolarizing current steps (control: n = 13 (6 mice), F(1, 12) = 11.36, **p = 0.0056; CRS: n = 11 (6 mice), RM ANOVA F(1, 10) = 4.112, p = 0.0701). All the data presented as mean ± SEM. Data on resting membrane potential (Vm) and properties of the action potentials (AP) (rheobase, threshold and half-width) for LA PV interneurons in control and CRS-exposed mice is shown in Supplementary Data 2.
The density of Grik1 expressing neurons in the LA was not significantly different between CRS-exposed and control mice (Fig. 4B). Also, the relative density of the Pvalb expressing GABAergic neurons in the LA was not affected by CRS treatment (Fig. 4B). However, the percentage of Pvalb neurons co-expressing Grik1 was significantly lower in CRS group as compared to the controls, while no difference in Grik1 expression was detected in other subtypes of GABAergic neurons expressing Gad1 but not Pvalb (Fig. 4C).
For further insight into the effect of chronic stress on Grik1 expression levels, the intensity of Grik1 ISH signal in individual cells was quantified by counting the number of dots representing Grik1 mRNA staining. The mean intensity of Grik1 ISH staining in Pvalb positive cells was substantially lower in the CRS group as compared to controls (Fig. 4D). Again, this difference was not observed in other subtypes of GABAergic neurons expressing Gad1 but not Pvalb (Fig. 4D). These data indicate that chronic stress results in downregulation of Grik1 expression selectively in LA PV interneurons.
To test whether the observed downregulation of Grik1 expression was sufficient to perturb GluK1 KAR function, we did current clamp experiments in LA PV interneurons in acute slices from control and CRS-exposed PV-TdTom mice. As shown previously [29], KAR antagonism by ACET resulted in significant attenuation of the PV interneuron firing rate in response to depolarizing current steps in slices from control mice (Fig. 4E, F), but not in mice lacking GluK1 selectively in PV interneurons (PV-Cre::Grik1fl/fl)(Supplementary Fig. 3A). In CRS-exposed mice, the basal excitability of the PV interneurons was lower as compared to the controls, and ACET application had no effect on the firing frequency (Fig. 4E, F). To confirm this result, we also used another, structurally distinct GluK1 antagonist, LY382884 [39]. Similarly to ACET, LY382884 (10 µM) attenuated PV interneuron firing rate in response to depolarizing current steps in slices from control, but not in CRS exposed PV-TdTom mice (Supplementary Fig. 3B, C). Interestingly, the low PV excitability after both CRS and Grik1 ablation was associated with high rheobase (Supplementary Data 2) [40]. These data indicate that CRS results in loss of functional KARs and reduced excitability in LA PV interneurons.
Chronic stress regulates LA output to CeL in a cell-type specific manner
LA principal neurons have direct and indirect projections to the centrolateral amygdala (CeL) [41], containing functionally distinct subpopulations of GABAergic neurons that can be identified based on expression of specific marker proteins (SOM, PKCδ and CRF) [42]. Plastic changes in the intra-amygdaloid connectivity can define activation of distinct output pathways underlying fear-related behaviors [43, 44], yet it remains unclear how the circuitry is modulated by chronic stress. To understand how LA hyperexcitability in response to loss of the GluK1-GABAB-mediated tonic inhibition and chronic stress relates to LA output to CeL, we focused on the connections to the CeL PKCδ expressing (PKCδ+) neurons implicated in the regulation of anxiety-like behaviors [15, 16]. Previous work indicates that connectivity from the lateral-basolateral amygdala complex to CeL is preserved in a coronal slice preparation [14, 45].
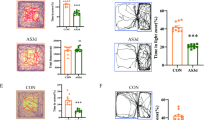
Recordings of spontaneous glutamatergic activity (sEPSCs) in slices from PKCδ-TdTom mice indicated that the basal sEPSC frequency in PKCδ+ neurons was higher in CRS-exposed mice as compared to controls, while in the surrounding PKCδ-CeL neurons, the sEPSC frequency was not significantly affected by the CRS treatment (Fig. 5A). We also analyzed the frequency of sIPSCs from the same recordings (Supplementary Fig. 4A) but found no differences between the groups. CeL neurons receive glutamatergic inputs from several brain areas. To test whether the observed increase in the sEPSC frequency in the PKCδ+ neurons depended on glutamatergic inputs from BLA, we used chemogenetic tools to selectively inhibit activity of BLA PN neurons in control and CRS-exposed mice (Fig. 5B, C). CNO application in acute slices from mice expressing the inhibitory DREADD receptor hM4Di in the BLA PNs resulted in 49 ± 8% reduction in the sEPSC frequency in PKCδ+ neurons, indicating that BLA neurons significantly contribute to the excitatory drive to PKCδ+ neurons. Interestingly, when the same experiment was repeated in CRS-exposed mice, the % inhibition of the sEPSC frequency in response to CNO application was higher than in controls (71 ± 5%, t-test vs control group, p = 0.046). In the presence of CNO, sEPSC frequency in PKCδ+ neurons was not different between the control and CRS groups (Fig. 5D). These data indicate that chronic stress shifts the balance of excitatory drive from BLA towards PKCδ+ cells in the CeL.
A Voltage clamp recordings of sEPSCs from PKCδ+ and PKCδ- neurons in CeL, in acute slices from control and CRS-exposed PKCδ-TdTom mice. Pooled data on the sEPSC frequency in the two cell types (PKCδ+: control, n = 19 (7 mice), CRS, n = 16 (5 mice), t-test, t = 3.302, df = 33, **p = 0.0023; PKCδ-: control, n = 9 (4 mice), CRS, n = 9 (5 mice), t-test, t = 1.848, df = 6, p = 0.1140). B The experimental approach for chemogenetic inhibition of the BLA PNs in PKCδ-Cre mice. AAV viral vectors encoding for inhibitory DREADD receptor hM4Di under the CaMKII promoter were injected into the BLA to target PNs. PKCδ+ neurons in the CeL were visualized by injection of AAV viral vectors encoding Cre-dependent EGFP. C The effect of CNO (10 μM) on resting membrane potential (RMP, recorded under whole cell current clamp) and spontaneous AP firing (cell-attached recording) in hM4Di expressing BLA PNs. CNO application resulted in 6.4 ± 1.8 mV hyperpolarizing shift in the membrane potential and 28 ± 10% reduction in the frequency of spontaneous AP firing (RMP: n = 6 (3 mice), paired t-test, t = 3.468, df = 5, *p = 0.0179; AP : n = 13 (3 mice), one sample t-test, t = 2.655, df = 12, *p = 0.021). D Examples of sEPSC recordings from PKCδ+ neurons in slices from control and CRS-exposed mice, expressing the inhibitory DREADD receptor hM4Di in the BLA PNs, at control conditions and in the presence of CNO (10 µM). Pooled data on the effect of CNO on sEPSC frequency (control: baseline, n = 11, CNO, n = 10 (4 mice), paired t-test, t = 2.746, df = 19, *p = 0.0128; CRS: baseline, n = 10, CNO, n = 8 (4 mice), paired t-test, t = 4.493, df = 16, ***p = 0.0004). Comparisons between control vs. CRS: unpaired t-test, t = 3.836, df = 19, **p = 0.0011; control + CNO vs. CRS+CNO: unpaired t-test, t = 1.204, df = 16, p = 0.2463. E Example traces and pooled data on sEPSC recordings from PKCδ+ neurons before and after application of ACET (200 nM) in control and CRS-exposed animals (control: n = 12 (5 mice), paired t-test, t = 4.452, df = 11, ***p = 0.001; CRS: n = 7 (5 mice), paired t-test, t = 0.7174, df = 6, p = 0.50). F Examples of sEPSC recordings from PKCδ+ neurons before and after application of ACET (200 nM) in mice expressing hM4Di in the BLA, in absence or continuous presence of CNO. Pooled data on the effect of ACET on sEPSC frequency (hM4Di: n = 6 (4 mice), paired t-test, t = 4.881, df = 5, **p = 0.0045; hM4Di + CNO: n = 6 (4 mice), paired t-test, t = 1.447, df = 5, p = 0.2074). All the data presented as mean ± SEM.
Finally, we investigated whether the loss of GluK1 KARs, regulating the excitability of the LA PNs via tonic inhibition, contributed to the increase in the sEPSC frequency in PKCδ+ neurons after chronic stress. To this end, we tested the effect of GluK1 antagonist ACET on sEPSCs in PKCδ+ neurons in control and CRS-exposed mice, and during DREADD-mediated inhibition of BLA neurons. Application of ACET resulted in a significant increase in sEPSC frequency in PKCδ+ neurons in the control group but had no effect when the same experiment was performed after CRS treatment (Fig. 5E). Furthermore, ACET application during chemogenetic inhibition of BLA PNs had no effect on sEPSC frequency in PKCδ+ neurons (Fig. 5F), suggesting that the ACET-induced increase in glutamatergic drive to PKCδ+ neurons depends on the activity of BLA PNs. Since KARs are implicated in regulation of glutamate release in the BLA-CeL synapses [46], particularly in young animals [47], we also tested whether pharmacological manipulation of GluK1 KARs affected glutamate release probability by recording the paired-pulse facilitation ratio (PPR) of EPSCs in PKCδ+ neurons in response to stimulation of the LA. However, in contrast to the neonatal animals [47], application of GluK1 agonist ATPA or antagonist ACET had no effect on PPR in the adult mice (Supplementary Fig. 4B). Together, these data are consistent with a model where stress-induced loss of the GluK1–GABAB-mediated tonic inhibition results in hyperexcitability of the LA PNs and facilitates glutamatergic drive selectively to the PKCδ+ neurons in the CeL.
Mice lacking Grik1 expression in PV interneurons are resistant to CRS-induced alterations in amygdala excitability and behavior
In order to understand the significance of the GluK1-GABAB-mediated regulation in stress-induced amygdala hyperexcitability and anxiety-like behaviors, we exposed the mice lacking GluK1 expression selectively in PV interneurons (PV-Cre::Grik1fl/fl) together with littermate controls (Grik1fl/fl) to chronic restraint stress (CRS). Both genotypes showed a significant loss of weight during the protocol, indicative of a physiological response to the stress (Fig. 6A).
A Body weight changes during chronic restraint stress (CRS) protocol (Grik1fl/fl: control n = 17, CRS n = 19; RM ANOVA, F (1, 34) = 21.32, ****p < 0.0001; PV-Cre::Grik1fl/fl: control n = 19, CRS n = 17; RM ANOVA; F (1, 34) = 23.89, ****p < 0.0001). B Action potential frequencies in response to depolarizing current steps recorded from brain slices of control and CRS-treated, Grik1fl/fl or PV-Cre::Grik1fl/fl mice (Grik1fl/fl: control, n = 25 (7 mice), CRS, n = 17 (6 mice), RM ANOVA, F (1, 40) = 4.775, *p = 0.035; PV-Cre::Grik1fl/fl: control, n = 19 (6 mice), CRS, n = 14 (4 mice), RM ANOVA, F (1, 31) = 0.0001180, p = 0.99). C Tonic GABAB currents recorded from LA PNs in slices from control and CRS-treated, Grik1fl/fl or PV-Cre::Grik1fl/fl mice. All recordings were done at −50 mV holding potential in the presence of 50 μM of D-AP5, 200 μM picrotoxin, and 50 μM GYKI 53655 to block NMDA, GABAA, and AMPA receptors, respectively. The amplitude of the tonic current was measured as a change in the holding current in response to the application of 10 μM CGP55845. The graph illustrates averaged data on the amplitude of the tonic GABAB currents under various conditions (Grik1fl/fl: control, n = 15 (5 mice), CRS, n = 11 (3 mice); PV-Cre::Grik1fl/fl: control, n = 15 (4 mice), CRS, n = 18 (3 mice); two-way ANOVA for genotype and condition, genotype effect F(1,54) = 19.93, ****p < 0.0001; post-hoc comparison *** p = 0.0003, Bonferroni). D Current clamp recordings from PV interneurons in acute slices from control and CRS-exposed PV-Cre::Grik1fl/fl mice. Example traces illustrate the response of the PV neurons to depolarizing current steps (75 and 200 pA), for control and CRS groups. Pooled data on the action potential frequencies in response to depolarizing current steps (control, n = 12 (3 mice), CRS, n = 12 (3 mice), Mixed-effects model (REML) F(1, 22) = 1.157, p = 0.2938). E Results of open field (OF) test. The graphs show the total time spent in the center area of the open field (OF) and the center time in 5 min bins, for control and CRS-exposed mice for the two genotypes, Grik1fl/fl and PV-Cre::Grik1fl/fl (Grik1fl/fl: control, n = 16, CRS, n = 19; PV-Cre::Grik1fl/fl: control, n = 19, CRS, n = 17). Total time in the center field: Two-way ANOVA: treatment F(1, 67) = 3.086, p = 0.0835, genotype F(1, 67) = 0.7517, p = 0.389, interaction F(1, 67) = 4.133, p = 0.046. * p = 0.019, Holm-Sidak. Time in center zone in 5 min bins: RM ANOVA, Grik1fl/fl: F(1, 34) = 8.564, **p = 0.0062; PV-Cre::Grik1fl/fl : F (1, 34) = 0.03320, p = 0.8566. Total distance traveled: 2-way ANOVA, genotype F(1,67) = 31.74, ****p < 0.0001, treatment F(1, 67) = 1.828, p = 0.1780, interaction F(1, 67) = 1.495, p = 0.2442. All the data are presented as mean ± SEM.
The excitability of LA PNs was investigated using current clamp recordings in brain slices from control and CRS-exposed mice of both genotypes. As expected, in the Grik1fl/fl mice, the firing rate of the LA PNs in response to depolarizing current steps was higher in slices from CRS-exposed mice as compared to controls (Fig. 6B). In contrast, CRS had no effect on the firing rate of LA PNs in the PV-Cre::Grik1fl/fl mice lacking GluK1 KARs in PV neurons (Fig. 6B). Furthermore, while in the Grik1fl/fl mice the CGP55845 sensitive tonic GABAB currents were smaller in the CRS group as compared to controls, these currents were very low in amplitude in the PV-Cre::Grik1fl/fl mice and not significantly different between control and CRS groups (Fig. 6C). These data suggest that LA PNs in PV-Cre::Grik1fl/fl mice are resistant to CRS induced hyperexcitability because they lack tonic GABAB receptor-mediated inhibition.
We also studied the effects of CRS on the excitability of PV interneurons in the PV-Cre::Grik1fl/fl mice. In these experiments, PV interneurons were identified based on expression of virally delivered Cre-dependent EGFP. As reported previously [40], the PV interneuron firing rate in response to depolarizing current steps in the PV-Cre::Grik1fl/fl mice was lower than in WTs (F (1, 22) = 6323, p = 0.019; 2-way RM ANOVA). However, the firing rate was not different between control and CRS groups (Fig. 6D). Also, in contrast to controls, in the PV-Cre::Grik1fl/fl mice, CRS treatment had no effect on the rheobase of the PV interneurons (Supplementary Data 2). Together with the previous data (Fig. 4), these results suggest that the reduced excitability of LA PV interneurons after CRS depends on GluK1 KARs.
The anxiety-like behavior was tested using an open field test (OF). The CRS treatment in the controls (Grik1fl/fl) resulted in the anticipated increase in anxiety-like behavior, involving avoidance of the open space in the center area of the arena (Fig. 6E). In contrast, the CRS-exposed PV-Cre::Grik1fl/fl mice behaved similarly as their littermate controls and there were no differences between the groups in the time spent in the center area of the open field arena (Fig. 6E). In this cohort of mice, the locomotor activity was not affected by CRS treatment; however, we observed that the PV-Cre::Grik1fl/fl mice were more active as compared to their littermates, indicated by the total distance traveled in the OF arena during the test (Fig. 6E).
Discussion
Changes in GABAergic inhibition contribute to stress-induced anxiety in rodents [16, 23, 24, 48], yet the detailed molecular and circuit mechanisms involved are not fully understood. Here we show that chronic restraint stress attenuates tonic GABAB receptor-mediated inhibition of LA PNs, resulting in their hyperexcitability and perturbed output to CeL neurons controlling anxiety-like behaviors. Furthermore, we show that this mechanism is regulated by GluK1-driven GABA release from PV interneurons, identifying PV interneurons and GluK1 receptors as critical gatekeepers of the stress-sensitive circuits in the amygdala.
Release of tonic GABAB receptor-mediated inhibition in amygdala after chronic stress
Previously, anxiolytic effects of GABAB agonists, such as baclofen, have been described in both humans and rats, and prominent anxiety-like behaviors have been reported in mice lacking GABAB receptor subunits [49, 50]. Our present data provides mechanistic explanation for these findings, and directly links endogenous tonic activity of GABAB receptors to amygdala excitability.
Weakening of tonic inhibition can be mediated by downregulation of the postsynaptic / extrasynaptic GABA receptors or reduction in the levels of ambient extracellular GABA. While chronic stress alters the expression of various GABAA receptor subunits [51] and in particular, the extrasynaptic GABAAδ and α5 subunits in the amygdala [16, 24], no changes in the postsynaptic response to GABAB agonists has been observed in response to stress [24, 48]. Instead, we observed that chronic stress was associated with downregulation of GluK1 subunit containing kainate receptors facilitating both action potential-dependent and asynchronous GABA release in PV interneurons. Selective genetic ablation or pharmacological inhibition of these receptors substantially reduced the tonic GABAB receptor-mediated inhibition of LA PNs. These results support that the stress-induced weakening of the tonic GABAB currents in LA PNs depends on changes in ambient GABA, due to loss of GluK1 KARs facilitating GABA release from PV interneurons.
PV interneurons have a well-characterized role in pacing and synchronizing activity of the principal neurons [8]. Intriguingly, our data show that alterations in PV interneuron physiology can also induce sustained changes in PN excitability via GABAB receptor-mediated tonic inhibition and this is critical for LA hyperexcitability after chronic stress. This finding may have broader significance for mechanistic understanding of the various neurological and psychiatric conditions involving PV interneuron dysfunction and altered circuit excitability [52, 53].
GluK1 KARs in PV interneurons regulate amygdala excitability
GluK1 KARs have been previously implicated in stress-induced alterations in amygdala as well as in anxiety-like behaviors [28, 29, 54]. However, since GluK1 KARs are expressed in both glutamatergic and GABAergic neurons in the amygdala and are found in different subtypes of GABAergic interneurons [36,37,38, 55], the cell types mainly responsible for GluK1-dependent modulation of amygdala excitability and amygdala dependent behaviors have remained unclear. Our present and previous [29, 40] results provide compelling data showing that GluK1 KARs are physiologically activated in LA PV interneurons to regulate their excitability and GABA release, which is critical for gating the excitability of the behaviorally relevant circuits in the amygdala. Accordingly, the absence of this regulation in mice lacking Grik1 expression in PV interneurons was associated with resilience to stress-induced anxiety.
In a physiological context, the GluK1–GABAB interplay may operate as a feedback system restricting excitability of the LA PNs during intense glutamatergic activity. In the immature hippocampus, GluK1 KARs are physiologically activated by ambient extracellular glutamate in both, principal neurons and GABAergic interneurons in slice preparations [56, 57]. Assuming similar mechanism in the amygdala, accumulation of extracellular glutamate during strong synchronous excitatory activity would result in enhanced activation of GluK1 receptors in PV interneurons, facilitating GABA release and GABAB-receptor mediated tonic inhibition of the principal neurons. In slice preparations, as in the present study, the effect of KAR antagonism on PV interneuron firing rate was relatively small. It is plausible, however, that this mechanism has more power in intact neural networks, with higher concentrations of ambient glutamate. Hence, together with previous findings reviewed in [58, 59], our data support that GluK1 KARs mediate crosstalk between glutamatergic and GABAergic systems to control network excitability. In addition to regulating the overall excitability, GluK1 KARs in PV interneurons could also affect fast synchronization of the amygdala circuity [60, 61] implicated in memory consolidation [1]. How exactly GluK1 KARs regulate activity of PV interneurons and consequently, information transfer in amygdala networks remains an interesting target for future studies.
Stress-induced target-specific plasticity in the LA-CeL circuitry
One of the main targets of BLA principal neurons is centrolateral amygdala (CeL), which is composed of intermingled populations of functionally distinct GABAergic neurons strongly implicated in regulation of anxiety and fear generalization [14, 42, 62]. BLA inputs to CeL undergo target-specific plasticity during fear acquisition and extinction [43, 44, 63], affecting the relative weights of excitatory input to different CeL neuron populations. Interestingly, while dynamic modulation of the connectivity is critical for fear memory acquisition and retrieval, persistent unbalance in the cell-type specific BLA–CeL connectivity is associated with anxiety–like behaviors in genetically modified mouse models [46]. In our experiments, anxiogenic chronic stress resulted in a shift in glutamatergic transmission from BLA towards CeL PKCδ+ neurons. Since activity of PKCδ+ neurons has been directly linked to anxiety-like behaviors in rodents [15, 16], these data suggest that the specific increase in the excitability of the LA-CeL PKCδ circuit critically contributes to stress-induced anxiety. However, when discussing stress pathophysiology, it should be noted that stress widely affects the brain and modulates various networks integrating external and internal factors, which can also contribute to the behavioral outcome.
Materials and methods
Animals
C57BL/6N-Grik1tm1c(KOMP)Mbp (Grik1fl/fl; [47]) mouse line was crossed with a B6.129P2-Pvalbtm1(cre)Arbr/J (PV-Cre) (JAX 008069) line to produce ablation of Grik1 gene selectively in the parvalbumin interneurons (PV-Cre::Grik1fl/fl). PKCδ-Cre mouse line (C57Bl/6CRL-Prkcd-iCre (GENSAT 011559-UCD), kindly provided by Wulf Haubensak) was crossed with Ai14 tdTomato reporter (JAX 007914) to visualize PKCδ cells in the central amygdala. PV-Cre mice were crossed with Ai14 tdTomato reporter to visualize parvalbumin interneurons in BLA. The mice were group housed in individually ventilated cages with a 12 light/12 dark cycle (lights are off 7:00 p.m. – 7:00 a.m.), and food and water were supplied ad libitum. All animal experiments were performed by following the University of Helsinki Animal Welfare Guidelines and approved by the National Animal Experiment Board of Finland (license numbers: KEK-17-019, KEK22-010, ESAVI/29384/2019, and ESAVI/31984/2022). Three-month-old male C57BL/6JRccHsd mice were used for ISH. Two- to three-month-old male mice were used for behavioral testing and electrophysiology.
Chronic restraint stress (CRS)
Mice were randomly assigned into two groups: the CRS group and the control group. Littermates were placed equally in both groups. The mice in both groups were weighed daily at the same time before starting the CRS procedure. The mice belonging to the CRS group were restrained in well-ventilated 50 ml Falcon tubes at the same time each day (8:30 a.m.–9:30 a.m.) for 10 consecutive days. During the restraining period, the control mice remained in their home cages in the same room where the restraint stress procedure took place. Food and water were supplied ad libitum in home cages.
Behavioral testing
The open field (OF) test was done on the 11th day of the experiment (after the 10 days CRS protocol). The test was done in a room with dimmed lights, in four 50 cm × 50 cm arenas with 32 cm high walls, with one mouse in each arena per trial. At the beginning of each trial, one mouse was placed in the corner of each arena and observed for 15 min using EthoVision XT15 (Noldus, Wageningen, Netherlands) video-tracking software. At the end of each trial, the arena was cleaned with 70% EtOH and wiped dry to remove any scent traces. In all behavioral tests, the person performing the experiments and extracting the data was blind to the genotype
Viral injections
AAV viral vectors encoding for inhibitory DREADD (AAV8, pAAV-CaMKIIa-hM4D(Gi)-mCherry; Addgene #50477) and/or floxed EGFP (AAV8; pAAV-hSyn-DIO-EGFP; Addgene #50457) were bilaterally injected to the BLA of adult (>P55) heterozygote PKCδ-Cre or PV-Cre::Grik1fl/fl mice. AAV viral vector encoding the Cre-activated expression of ChR2 (AAV1; pAAV-EF1a-double floxed-hChR2(H134R)-mCherry-WPRE-HGHpA; Addgene #20297) was injected bilaterally in the BLA of adult heterozygote PV-Cre mice. Injections were done under anesthesia in a stereotaxic frame as described [47], using the following coordinates for BLA (from bregma): (1) Ap −1,8 ML 3.4–3.5 DV 4.1–4.3 (2) Ap −2.3 ML 3.4–3.5 DV 4.1–4.3; and for CeA: AP 1.8 ML 2.9 DV 4.4.
Electrophysiology
Acute slices
Acute coronal sections were prepared as previously [29]. Briefly, the brain was removed and immediately placed in carbonated (95% O2/ 5% CO2) ice-cold N-Methyl-D-glucamine (NMDG) based protective cutting solution (pH 7.3–7.4) containing (in mM): 92 NMDG, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 Na-pyruvate, 0.5 CaCl2 and 10 MgSO4. The vibratome (Leica VT 1200S) was used to obtain 300-µm-thick brain slices. Slices containing the amygdala were placed into a slice holder and incubated for 8–10 min in 34 °C in the NMDG–based solution. Slices were then transferred into a separate slice holder at room temperature with a solution containing (in mM): 92 NaCl, 2.5 KCl, 1.25 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 2 thiourea, 5 Na-ascorbate, 3 Na-pyruvate, 2 CaCl2 and 2 MgSO4 (saturated with 95% O2/5% CO2).
Whole-cell recordings
After 1–4 h of recovery, the slices were placed in a submerged heated (30–32 °C) recording chamber and continuously perfused with standard ACSF, containing (in mM): 124 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 15 glucose, 1 MgSO4 and 2 CaCl2, at the speed of 1.5–2 ml/min. Whole-cell patch-clamp recordings were done from amygdala neurons under visual guidance using glass capillary microelectrodes with resistance of 3–5.5 MΩ. Multiclamp 700B amplifier (Molecular Devices), Digidata 1322 (Molecular Devices) or NI USB-6341 A/D board (National Instruments), and WinLTP version 2.20 [64] or pClamp 11.0 software were used for data collection, with low pass filter (10 kHz) and a sampling rate of 20 kHz. In voltage-clamp recordings, uncompensated series resistance (Rs) was monitored by measuring the peak amplitude of the fast whole-cell capacitance current in response to a 5 mV step. Only experiments where Rs <30 MΩ, and with <20% change in Rs during the experiment, were included in the analysis.
Whole-cell current clamp recordings of membrane excitability in principal neurons and PV interneurons in LA were performed using a filling solution containing (in mM): 135 K-gluconate, 10 HEPES, 5 EGTA, 2 KCl, 2 Ca(OH)2, 4 Mg-ATP, 0.5 Na-GTP, (280 mOsm, pH 7.2). The resting membrane potential was sampled and then adjusted to −70 mV. Depolarizing current steps with 600 ms (PV interneurons) or 1000 ms duration (principal neurons) were applied to induce action potential firing. The amplitude of the injected current was increased with 10–20 pA increments. Since the principal neuron firing rate is not stable under whole-cell recording conditions, the excitability data was always collected immediately after whole-cell access, under control conditions or in the presence and after > 10 min preincubation of a relevant antagonist (200 nM ACET, 5 µM CGP55845). The effect of GluK1 antagonism on PV interneuron excitability was addressed by using fast application of 200 nM ACET in the continuous presence of 50 µM GYKI 53655, 5 µM L-689560 and 100 µM picrotoxin, to block AMPA, NMDA and GABAA receptors.
Drug-induced GABAA- and GABAB-mediated currents were recorded from LA PNs in the presence of antagonists for NMDA and AMPA receptors (50 µM AP-5 and 50 µM GYKI 53655, respectively). GluK1 antagonist ACET (200 nM) was included in some of the experiments. For recordings of GABAB receptor-mediated currents, the intracellular solution contained (in mM): 140 K-gluconate, 10 HEPES, 1 EGTA, 2 KCl, 2 NaCl, 4 Mg-ATP, 0.5 Na-GTP, (280 mOsm, pH 7.2), 100 µM picrotoxin was included in the ACSF to block GABAA receptors, and the holding potential of the neurons was −50 mV. For recordings of GABAA-mediated currents, the intracellular solution contained (in mM): 130 CsCl, 10 HEPES, 0.5 EGTA, 8 NaCl, 4 Mg-ATP, 0.3 Na-GTP, 5 QX314 (280 mOsm, pH 7.2), 5 µM CGP 55845 hydrochloride was included in the ACSF to block GABAB receptors, and the holding potential of the neurons was −90 mV.
A valve control system (VC-6-PINCH, Warner Instruments) with a manifold adjusted to the tube was used for fast drug application. ACSF including the above antagonists was applied directly to the LA to record a baseline before switching to solution supplemented with the relevant drug (10 µM CGP55845 hydrochloride, 25 µM SKF97541 or 25 µM (-)-Bicuculline methochloride).
mAHP currents
Medium duration afterhyperpolarizing (mAHP) currents were recorded from LA PNs in voltage clamp mode at holding potential −50 mV, using filling solution containing (in mM): 135 K-gluconate, 10 HEPES, 5 EGTA, 2 KCl, 2 Ca(OH)2, 4 Mg-ATP, 0.5 Na-GTP, (280 mOsm, pH 7.2). mAHP currents were induced by 50 ms depolarization step from −50–0 mV.
Spontaneous synaptic currents
sEPSCs and sIPSCs in PKCδ neurons and LA principal neurons were recorded under whole-cell voltage-clamp with a filling solution containing (in mM): 135 K-gluconate, 10 HEPES, 5 EGTA, 2 KCl, 2 Ca(OH)2, 4 Mg-ATP, 0.5 Na-GTP, (280 mOsm, pH 7.2) at holding potential of −50 mV. ACET (200 nM) and CNO (10 µM) were added to the ACSF. For more detailed analysis of sIPSCs in LA principal neurons and for light-induced IPSCs, recordings were done using high-chloride intracellular solution containing (in mM): 130 CsCl, 10 HEPES, 0.5 EGTA, 8 NaCl, 4 Mg-ATP, 0.3 Na-GTP, 5 QX314 (280 mOsm, pH 7.2), at −80 mV holding potential.
Evoked EPSCs were recorded in from PKCδ neurons in CeA at a holding potential of −70 mV, using electrodes filled with the following solution (in mM): 130 CsMeSO4, 10 HEPES, 0.5 EGTA, 8 NaCl, 4 Mg-ATP, 0.3 Na-GTP, 5 QX314 (280 mOsm, pH 7.2). Picrotoxin (100 µM) and D-AP5 (50 µM) were included in the perfusion solution to block fast GABAA and NMDAR-mediated components of synaptic transmission, respectively. EPSCs were evoked with bipolar stimulation electrodes (nickel-chromium wire), placed at LA to mimic the activation of glutamatergic LA afferents. Paired-pulse responses (PPR) were evoked with the inter-pulse interval of 50 ms.
Optical stimulation of PV interneurons was done using pE-2 LED system (CoolLED, UK). Short pulses (0.2–0.5 ms, 50 ms interval) of 470 nm blue light were used for paired-pulse stimulation. Asynchronous release was triggered with 750 ms long light pulses. LED intensity was kept constant throughout the recording.
Cell attached recordings
Spontaneous action potentials were recorded from LA principal neurons in cell attached configuration in voltage clamp mode. 150 mM NaCl was used as filling solution. Membrane integrity during the experiment was monitored via square 5 mV step every 30 seconds of the recording.
Data analysis
WinLTP software [64] was used to calculate the peak amplitude of the evoked synaptic responses. For analysis of paired-pulse ratio (PPR), 7–10 responses were averaged in each experimental condition. PPR was calculated as the amplitude ratio of response 2/response 1. The frequency and amplitude of spontaneous synaptic events were analyzed using miniAnalysis program 6.0.3. sIPSCs and sEPSCs were identified in the analysis as outward or inward currents (for IPSCs depending on experimental conditions) with typical kinetics, respectively, that were at least 3 times the amplitude of the baseline level of noise. For kinetic measurements, the decay time constant τ (decay to 37% of peak amplitude) was determined by fitting a single exponential curve to the decay phase of the IPSC. Event decay time distribution was then plotted with 1 ms bins as % of total number of events. For the pooled data, averages for baseline, drug application and washout were calculated over a 10-minute period. Action potential frequencies were analyzed using the threshold search algorithm in Clampfit software. AP half-width and amplitude of the fast hyperpolarizing potential were analyzed from the 3rd spike in the train using Clampfit software.
In situ hybridization
Triple-ISH was done using the RNAscope® Fluorescent Multiplex kit (Advanced Cell Diagnostics) on fresh frozen brain sections from control and CRS-exposed mice. The brain was removed under anesthesia, flash frozen in dry ice and cut into 10 µm-thick coronal sections with a cryostat (Leica CM3050). The sections were dried in the cryostat at −20 °C for 1 h, after which they were stored at −80 °C until the next day. Before hybridization, the sections were fixed in cold 4% PFA for 30 min, dehydrated in ethanol series, and treated with protease for 30 min at room temperature (RT). The hybridization reaction was performed according to the manufacturer’s instructions, using the following probes: Mm-Pvalb-C1, Mm-GAD1-C2, and Mm-Grik1-C3 (Advanced Cell Diagnostics). After hybridization, the slices were stained with DAPI. The sections containing the BLA were imaged with Zeiss Axio Imager.M2 microscope equipped with ApoTome 2 attachment, using PlanApo ×20 and ×40 objectives, CMOS camera (Hamamatsu ORCA Flash 4.0 V2), and Zen 2 software. The density of stained cells within the brain region of interest were analyzed from ×20 images using ImageJ v1.53 f software. The threshold for Grik1 expression was assigned to >2 dots co-localized with a cell marker. The signal intensity of Grik1 expression at the single-cell level was quantified by counting the number of puncta per cell semi-automatically with QuPath software v0.3.2 [65] from ×40 images. All the data were obtained from at least 4 sections/animal (4 animals per group).
Statistical analysis
All statistical analyses were done on raw (not normalized) data using Graph Pad Prism 9.0 software. Sample size was based on previous experience on similar experiments. All data were first assessed for normality and homogeneity of variance and the statistical test was chosen accordingly. Differences between two groups were analyzed using two-tailed t-test or Mann-Whitney U test. Two-way ANOVA with Holm-Sidak post hoc comparison was used to compare effects of genotype and stress. For data that was not normally distributed, Mann-Whitney test was used for post-hoc pairwise comparison. Dunnett test was used for testing two or more experimental groups against a single control group. Holm-Sidak multiple t-test was used to identify the difference in % of IPSC events grouped by their decay time between stress and control groups, at control condition and in the presence of ACET, at baseline and after optogenetic stimulation. Kolmogorov-Smirnov test was used to compare distributions of Grik1-expressing parvalbumin interneurons in control and stressed animals’ amygdala. To compare drug effects to the baseline, Student’s paired two-tailed t-test was used. The results were considered significant when p < 0.05. All the pooled data are given as mean ± S.E.M.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information. Raw data are available from the corresponding author upon reasonable request.
References
Paré D, Headley DB. The amygdala mediates the facilitating influence of emotions on memory through multiple interacting mechanisms. Neurobiol Stress. 2023;24:100529.
Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33.
Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 2015;16:317–31.
Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–404.
Prager EM, Bergstrom HC, Wynn GH, Braga MF. The basolateral amygdala gamma-aminobutyric acidergic system in health and disease. J Neurosci Res. 2016;94:548–67.
Sharp BM. Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl Psychiatry. 2017;7:e1194.
Zhang WH, Zhang JY, Holmes A, Pan BX. Amygdala circuit substrates for stress adaptation and adversity. Biol Psychiatry. 2021;89:847–56.
Hu H, Gan J, Jonas P. Fast-spiking, parvalbumin+ GABAergic interneurons: from cellular design to microcircuit function. Science. 2014;345:1255263.
Babaev O, Piletti Chatain C, Krueger-Burg D. Inhibition in the amygdala anxiety circuitry. Exp Mol Med. 2018;50:1–16.
Woodward EM, Coutellier L. Age- and sex-specific effects of stress on parvalbumin interneurons in preclinical models: relevance to sex differences in clinical neuropsychiatric and neurodevelopmental disorders. Neurosci Biobehav Rev. 2021;131:1228–42.
Luo ZY, Huang L, Lin S, Yin YN, Jie W, Hu NY, et al. Erbin in amygdala parvalbumin-positive neurons modulates anxiety-like behaviors. Biol Psychiatry. 2020;87:926–36.
Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–71.
Polepalli JS, Gooch H, Sah P. Diversity of interneurons in the lateral and basal amygdala. NPJ Sci Learn. 2020;5:10.
Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–62.
Cai H, Haubensak W, Anthony TE, Anderson DJ. Central amygdala PKC-δ(+) neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci. 2014;17:1240–8.
Botta P, Demmou L, Kasugai Y, Markovic M, Xu C, Fadok JP, et al. Regulating anxiety with extrasynaptic inhibition. Nat Neurosci. 2015;18:1493–500.
Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–36.
Zhang WH, Liu WZ, He Y, You WJ, Zhang JY, Xu H, et al. Chronic stress causes projection-specific adaptation of amygdala neurons via small-conductance calcium-activated potassium channel downregulation. Biol Psychiatry. 2019;85:812–28.
Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. Increased basolateral amygdala pyramidal cell excitability may contribute to the anxiogenic phenotype induced by chronic early-life stress. J Neurosci. 2015;35:9730–40.
Wei J, Zhong P, Qin L, Tan T, Yan Z. Chemicogenetic restoration of the prefrontal cortex to amygdala pathway ameliorates stress-induced deficits. Cereb Cortex. 2018;2018:1980–90.
Liu WZ, Zhang WH, Zheng ZH, Zou JX, Liu XX, Huang SH, et al. Identification of a prefrontal cortex-to-amygdala pathway for chronic stress-induced anxiety. Nat Commun. 2020;11:2221.
Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29.
Liu ZP, Song C, Wang M, He Y, Xu XB, Pan HQ, et al. Chronic stress impairs GABAergic control of amygdala through suppressing the tonic GABAA receptor currents. Mol Brain. 2014;7:32.
Qin X, Pan HQ, Huang SH, Zou JX, Zheng ZH, Liu XX, et al. GABAA(δ) receptor hypofunction in the amygdala-hippocampal circuit underlies stress-induced anxiety. Sci Bull. 2022;67:97–110.
Mackay JP, Bompolaki M, DeJoseph MR, Michaelson SD, Urban JH, Colmers WF. NPY2 receptors reduce tonic action potential-independent GABAB currents in the basolateral amygdala. J Neurosci. 2019;39:4909–30.
Marron Fernandez de Velasco E, Tipps ME, Haider B, Souders A, Aguado C, Rose TR, et al. Ethanol-induced suppression of G protein-gated inwardly rectifying K+-dependent signaling in the basal amygdala. Biol Psychiatry. 2023;94:863–74.
Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala. J Neurosci. 2003;23:442–52.
Wu LJ, Ko SW, Toyoda H, Zhao MG, Xu H, Vadakkan KI, et al. Increased anxiety-like behavior and enhanced synaptic efficacy in the amygdala of GluR5 knockout mice. PLoS ONE. 2007;2:e167.
Englund J, Haikonen J, Shteinikov V, Amarilla SP, Atanasova T, Shintyapina A, et al. Downregulation of kainate receptors regulating GABAergic transmission in amygdala after early life stress is associated with anxiety-like behavior in rodents. Transl Psychiatry. 2021;11:538.
Dargan SL, Clarke VR, Alushin GM, Sherwood JL, Nisticò R, Bortolotto ZA, et al. ACET is a highly potent and specific kainate receptor antagonist: characterisation and effects on hippocampal mossy fibre function. Neuropharmacology. 2009;56:121–30.
Jane DE, Lodge D, Collingridge GL. Kainate receptors: pharmacology, function and therapeutic potential. Neuropharmacology. 2009;56:90–113.
Rodríguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901.
Jiang L, Xu J, Nedergaard M, Kang J. A kainate receptor increases the efficacy of GABAergic synapses. Neuron. 2001;30:503–13.
Cossart R, Tyzio R, Dinocourt C, Esclapez M, Hirsch JC, Ben-Ari Y, et al. Presynaptic kainate receptors that enhance the release of GABA on CA1 hippocampal interneurons. Neuron. 2001;29:497–508.
Maingret F, Lauri SE, Taira T, Isaac JT. Profound regulation of neonatal CA1 rat hippocampal GABAergic transmission by functionally distinct kainate receptor populations. J Physiol. 567. 2005;1:131–42.
Daw MI, Pelkey KA, Chittajallu R, McBain CJ. Presynaptic kainate receptor activation preserves asynchronous GABA release despite the reduction in synchronous release from hippocampal cholecystokinin interneurons. J Neurosci. 2010;30:11202–9.
Lourenço J, Cannich A, Carta M, Coussen F, Mulle C, Marsicano G. Synaptic activation of kainate receptors gates presynaptic CB(1) signaling at GABAergic synapses. Nat Neurosci. 2010;13:197–204.
Wyeth MS, Pelkey KA, Yuan X, Vargish G, Johnston AD, Hunt S, et al. Neto auxiliary subunits regulate interneuron somatodendritic and presynaptic kainate receptors to control network inhibition. Cell Rep. 2017;20:2156–68.
Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, et al. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301.
Haikonen J, Srinivasan R, Ojanen S, Rhee JK, Ryazantseva M, Zumaraite G, et al. GluK1 kainate receptors are necessary for functional maturation of parvalbumin interneurons regulating amygdala circuit function. Mol Psychiatry. 2024;29:3752–68.
Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–23.
Moscarello JM, Penzo MA. The central nucleus of the amygdala and the construction of defensive modes across the threat-imminence continuum. Nat Neurosci. 2022;25:999–1008.
Li H, Penzo MA, Taniguchi H, Kopec CD, Huang ZJ, Li B. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci. 2013;16:332–9.
Hartley ND, Gaulden AD, Báldi R, Winters ND, Salimando GJ, Rosas-Vidal LE, et al. Dynamic remodeling of a basolateral-to-central amygdala glutamatergic circuit across fear states. Nat Neurosci. 2019;22:2000–12.
Lopez de Armentia M, Sah P. Firing properties and connectivity of neurons in the rat lateral central nucleus of the amygdala. J Neurophysiol. 2004;92:1285–94.
Arora V, Pecoraro V, Aller MI, Román C, Paternain AV, Lerma J. Increased Grik4 gene dosage causes imbalanced circuit output and human disease-related behaviors. Cell Rep. 2018;23:3827–38.
Ryazantseva M, Englund J, Shintyapina A, Huupponen J, Shteinikov V, Pitkänen A, et al. Kainate receptors regulate development of glutamatergic synaptic circuitry in the rodent amygdala. Elife. 2020;9:e52798.
Pan HQ, Zhang WH, Liao CZ, He Y, Xiao ZM, Qin X, et al. Chronic stress oppositely regulates tonic inhibition in thy1-expressing and non-expressing neurons in amygdala. Front Neurosci. 2020;14:299.
Kumar K, Sharma S, Kumar P, Deshmukh R. Therapeutic potential of GABAB receptor ligands in drug addiction, anxiety, depression and other CNS disorders. Pharmacol Biochem Behav. 2013;110:174–84.
Felice D, O’Leary OF, Cryan JF. Targeting the GABAB receptor for the treatment of depression and anxiety disorders. In: Colombo G, editors. GABAB Receptor. Switzerland: Springer International Publishing; 2016. pp 219–50.
Maguire J. Stress-induced plasticity of GABAergic inhibition. Front. Cell.Neurosci. 2014;8:157.
Hijazi S, Smit AB, van Kesteren RE. Fast-spiking parvalbumin-positive interneurons in brain physiology and Alzheimer’s disease. Mol Psychiatry. 2023;28:4954–67.
Juarez P, Martínez Cerdeño V. Parvalbumin and parvalbumin chandelier interneurons in autism and other psychiatric disorders. Front Psychiatry. 2022;13:913550.
Masneuf S, Lowery-Gionta E, Colacicco G, Pleil KE, Li C, Crowley N, et al. Glutamatergic mechanisms associated with stress-induced amygdala excitability and anxiety-related behavior. Neuropharmacology. 2014;85:190–7.
Selvakumar P, Lee J, Khanra N, He C, Munguba H, Kiese L, et al. Structural and compositional diversity in the kainate receptor family. Cell Rep. 2021;37:109891.
Lauri SE, Vesikansa A, Segerstråle M, Collingridge GL, Isaac JT, Taira T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron. 2006;50:415–29.
Segerstråle M, Juuri J, Lanore F, Piepponen P, Lauri SE, Mulle C, et al. High firing rate of neonatal hippocampal interneurons is caused by attenuation of afterhyperpolarizing potassium currents by tonically active kainate receptors. J Neurosci. 2010;30:6507–14.
Lauri SE, Ryazantseva M, Orav E, Vesikansa A, Taira T. Kainate receptors in the developing neuronal networks. Neuropharmacology. 2021;195:108585.
Mulle C, Crépel V. Regulation and dysregulation of neuronal circuits by KARs. Neuropharmacology. 2021;197:108699.
Randall FE, Whittington MA, Cunningham MO. Fast oscillatory activity induced by kainate receptor activation in the rat basolateral amygdala in vitro. Eur J Neurosci. 2011;33:914–22.
Ojanen S, Kuznetsova T, Kharybina Z, Voikar V, Lauri SE, Taira T. Interneuronal GluK1 kainate receptors control maturation of GABAergic transmission and network synchrony in the hippocampus. Mol Brain. 2023;16:43.
Fadok JP, Markovic M, Tovote P, Lüthi A. New perspectives on central amygdala function. Curr Opin Neurobiol. 2018;49:141–7.
Terburg D, Scheggia D, Triana Del Rio R, Klumpers F, Ciobanu AC, Morgan B, et al. The basolateral amygdala is essential for rapid escape: a human and rodent study. Cell. 2018;175:723–35.e16.
Anderson WW, Collingridge GL. Capabilities of the WinLTP data acquisition program extending beyond basic LTP experimental functions. J. Neuroscience Methods. 2007;162:346–56.
Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD, et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7:16878.
Acknowledgements
We thank Vootele Voikar and the personnel of the Mouse Behavioral Phenotyping Facility and the Laboratory Animal Center for expert technical help. MR thanks Svetlana Molchanova for inspiring discussions. This study was financially supported by the Academy of Finland (#330710, SEL; #330298, MR) and Sigrid Juselius Foundation (#1158, SEL). Mouse Behavioral Phenotyping Facility and Helsinki Electrophysiology Platform are supported by Biocenter Finland.
Funding
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
Author information
Authors and Affiliations
Contributions
SEL and MR designed the study, MR, ML, VS and A-JK performed the electrophysiological experiments. ML performed the in situ hybridization, imaging and image analysis. MR, ML, JS, VS and A-JK carried out the CRS treatments. MR and JS performed the stereotaxic surgeries. SEL wrote the manuscript. All authors contributed to the manuscript editing and revisions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal experiments were conducted in accordance with the University of Helsinki Animal Welfare Guidelines and approved by the National Animal Experiment Board of Finland (license numbers: KEK-17-019, KEK22-010, ESAVI/29384/2019, and ESAVI/31984/2022).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ryazantseva, M., Liiwand, M., Shteinikov, V. et al. Parvalbumin interneurons gate amygdala excitability and response to chronic stress via kainate receptor-driven tonic GABAB receptor-mediated inhibition. Mol Psychiatry 30, 5093–5107 (2025). https://doi.org/10.1038/s41380-025-03093-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41380-025-03093-y