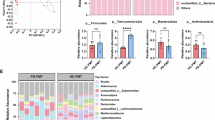
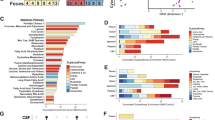
Abstract
Psychosocial stress in women is a major public health concern, yet the intergenerational mechanisms linking maternal premating stress to offspring neuropsychiatric vulnerability remain incompletely understood. Here, female mice were exposed to chronic unpredictable mild stress (CUMS) prior to mating, and offspring were assessed for neurodevelopment, adult behavior, and multi-omics profiles. Premating stress induced neurodevelopmental delays and sexually dimorphic adult phenotypes: female offspring exhibited hyperactivity and social deficits, whereas male offspring displayed anxiety. Cerebellum cytokine levels were reduced in a sex-dependent manner. Maternal stress shifted offspring gut microbiota (GM) composition, with p_Proteobacteria as core taxa in females and p_Firmicutes in males, exhibiting sex-dependent and inverse shifts in microbial network connectivity. Male offspring showed marked metabolic alterations and enhanced maternal–offspring metabolic concordance. Integrated analyses of GM, metabolites, and cerebellum profiles identified sexually dimorphic network correlations, further supported by human data demonstrating maternal stress-induced, sex-dependent GM network remodeling. Lactoferrin (LF) intervention selectively rescued male anxiety but not female behavioral deficits, and in males specifically, reduced cerebellum pro-inflammatory cytokines, enhanced GM network connectivity, and enriched immune-related serum metabolic and cerebellum transcriptomic pathways. LF also suppressed Purkinje cell firing frequency in males and reinforced post-treatment connectivity across microbial, metabolic, and cerebellum transcriptional nodes. Collectively, these findings delineate a previously unrecognized sexually dimorphic neuro–microbiota–metabolic network underpinning intergenerational vulnerability and highlight microbiota-targeted modulation as a systems-level mechanistic framework for sex-specific prevention of maternal stress–associated neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Analyzed data and images reported in this paper will be shared by the corresponding authors upon request.
References
Fu Q, Qiu R, Chen L, Chen Y, Qi W, Cheng Y. Music prevents stress-induced depression and anxiety-like behavior in mice. Transl Psychiatry. 2023;13:317.
Galley JD, Mashburn-Warren L, Blalock LC, Lauber CL, Carroll JE, Ross KM, et al. Maternal anxiety, depression and stress affects offspring gut microbiome diversity and bifidobacterial abundances. Brain Behav Immun. 2023;107:253–64.
Joffe JM. Genotype and prenatal and premating stress interact to affect adult behavior in rats. Science. 1965;150:1844–5.
Tegethoff M, Greene N, Olsen J, Schaffner E, Meinlschmidt G. Stress during pregnancy and offspring pediatric disease: a national cohort study. Environ Health Perspect. 2011;119:1647–52.
Faraji N, Payami B, Ebadpour N, Gorji A. Vagus nerve stimulation and gut microbiota interactions: a novel therapeutic avenue for neuropsychiatric disorders. Neurosci Biobehav Rev. 2025;169:105990.
Li TT, Chen X, Huo D, Arifuzzaman M, Qiao S, Jin WB, et al. Microbiota metabolism of intestinal amino acids impacts host nutrient homeostasis and physiology. Cell Host Microbe. 2024;32:661–75.e10.
Wallrapp A, Chiu IM. Neuroimmune interactions in the intestine. Annu Rev Immunol. 2024;42:489–519.
Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–12.
Fourie NH, Wang D, Abey SK, Creekmore AL, Hong S, Martin CG, et al. Structural and functional alterations in the colonic microbiome of the rat in a model of stress induced irritable bowel syndrome. Gut Microbes. 2017;8:33–45.
Ma J, Wang R, Chen Y, Wang Z, Dong Y. 5-HT attenuates chronic stress-induced cognitive impairment in mice through intestinal flora disruption. J Neuroinflammation. 2023;20:23.
Stilling RM, Ryan FJ, Hoban AE, Shanahan F, Clarke G, Claesson MJ, et al. Microbes & neurodevelopment-Absence of microbiota during early life increases activity-related transcriptional pathways in the amygdala. Brain Behav Immun. 2015;50:209–20.
Jasarevic E, Howard CD, Misic AM, Beiting DP, Bale TL. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci Rep. 2017;7:44182.
Malkova NV, Yu CZ, Hsiao EY, Moore MJ, Patterson PH. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav Immun. 2012;26:607–16.
Sun Y, Xie R, Li L, Jin G, Zhou B, Huang H, et al. Prenatal maternal stress exacerbates experimental colitis of offspring in adulthood. Front Immunol. 2021;12:700995.
Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7.
Ronan V, Yeasin R, Claud EC. Childhood development and the microbiome-the intestinal microbiota in maintenance of health and development of disease during childhood development. Gastroenterology. 2021;160:495–506.
Vuong HE, Pronovost GN, Williams DW, Coley EJL, Siegler EL, Qiu A, et al. The maternal microbiome modulates fetal neurodevelopment in mice. Nature. 2020;586:281–86.
Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med. 2017;23:314–26.
Choi H, Rao MC, Chang EB. Gut microbiota as a transducer of dietary cues to regulate host circadian rhythms and metabolism. Nat Rev Gastroenterol Hepatol. 2021;18:679–89.
Wastyk HC, Fragiadakis GK, Perelman D, Dahan D, Merrill BD, Yu FB, et al. Gut-microbiota-targeted diets modulate human immune status. Cell. 2021;184:4137–53.e14.
Yang M, Zheng X, Fan J, Cheng W, Yan TM, Lai Y, et al. Antibiotic-induced gut microbiota dysbiosis modulates host transcriptome and m(6)a epitranscriptome via bile acid metabolism. Adv Sci (Weinh). 2024;11:e2307981.
Jasarevic E, Morrison KE, Bale TL. Sex differences in the gut microbiome-brain axis across the lifespan. Philos Trans R Soc Lond B Biol Sci. 2016;371:20150122.
Wang S, Egan M, Ryan CA, Boyaval P, Dempsey EM, Ross RP, et al. A good start in life is important-perinatal factors dictate early microbiota development and longer term maturation. FEMS Microbiol Rev. 2020;44:763–81.
Berthon BS, Williams LM, Williams EJ, Wood LG. Effect of lactoferrin supplementation on inflammation, immune function, and prevention of respiratory tract infections in humans: a systematic review and meta-analysis. Adv Nutr. 2022;13:1799–819.
Abd El-Hack ME, Abdelnour SA, Kamal M, Khafaga AF, Shakoori AM, Bagadood RM, et al. Lactoferrin: antimicrobial impacts, genomic guardian, therapeutic uses and clinical significance for humans and animals. Biomed Pharmacother. 2023;164:114967.
Jamka M, Kaczmarek N, Mądry E, Krzyżanowska-Jankowska P, Bajerska J, Kręgielska-Narożna M, et al. Metabolic health in obese subjects-is there a link to lactoferrin and lactoferrin receptor-related gene polymorphisms? Nutrients. 2020;12:2843.
Chevalier G, Siopi E, Guenin-Mace L, Pascal M, Laval T, Rifflet A, et al. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat Commun. 2020;11:6363.
Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement Ther Clin Pract. 2020;39:101166.
Barak B, Feng G. Neurobiology of social behavior abnormalities in autism and Williams syndrome. Nat Neurosci. 2016;19:647–55.
Czarzasta K, Bogacki-Rychlik W, Segiet-Swiecicka A, Kruszewska J, Malik J, Skital V, et al. Gender differences in short- vs. long-term impact of maternal depression following pre-gestational chronic mild stress. Exp Neurol. 2022;353:114059.
Gruol DL, Calderon D, French K, Melkonian C, Huitron-Resendiz S, Cates-Gatto C, et al. Neuroimmune interactions with binge alcohol drinking in the cerebellum of IL-6 transgenic mice. Neuropharmacology. 2023;228:109455.
Topper LA, Baculis BC, Valenzuela CF. Exposure of neonatal rats to alcohol has differential effects on neuroinflammation and neuronal survival in the cerebellum and hippocampus. J Neuroinflammation. 2015;12:160.
Argaw-Denboba A, Schmidt TSB, Di Giacomo M, Ranjan B, Devendran S, Mastrorilli E, et al. Paternal microbiome perturbations impact offspring fitness. Nature. 2024;629:652–59.
Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99:1877–2013.
Koren O, Konnikova L, Brodin P, Mysorekar IU, Collado MC. The maternal gut microbiome in pregnancy: implications for the developing immune system. Nat Rev Gastroenterol Hepatol. 2024;21:35–45.
Matharu D, Ponsero AJ, Lengyel M, Meszaros-Matwiejuk A, Kolho KL, de Vos WM, et al. Human milk oligosaccharide composition is affected by season and parity and associates with infant gut microbiota in a birth mode dependent manner in a finnish birth cohort. EBioMedicine. 2024;104:105182.
Schirmbeck GH, Sizonenko S, Sanches EF. Neuroprotective role of lactoferrin during early brain development and injury through lifespan. Nutrients. 2022;14:2923.
Chen CH, Newman LN, Stark AP, Bond KE, Zhang D, Nardone S, et al. A Purkinje cell to parabrachial nucleus pathway enables broad cerebellar influence over the forebrain. Nat Neurosci. 2023;26:1929–41.
Babenko O, Kovalchuk I, Metz GA. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48:70–91.
Wehmer F, Porter RH, Scales B. Pre-mating and pregnancy stress in rats affects behaviour of grandpups. Nature. 1970;227:622.
Zoubovsky SP, Williams MT, Hoseus S, Tumukuntala S, Riesenberg A, Schulkin J, et al. Neurobehavioral abnormalities following prenatal psychosocial stress are differentially modulated by maternal environment. Transl Psychiatry. 2022;12:22.
Rinne GR, Davis EP, Mahrer NE, Guardino CM, Charalel JM, Shalowitz MU, et al. Maternal depressive symptom trajectories from preconception through postpartum: associations with offspring developmental outcomes in early childhood. J Affect Disord. 2022;309:105–14.
Wei Q, Zou J, Ma X, Xiao X, Zhang Y, Shi H. Prospective associations between various prenatal exposures to maternal psychological stress and neurodevelopment in children within 24 months after birth. J Affect Disord. 2023;327:101–10.
Xu Z, Zhang X, Chang H, Kong Y, Ni Y, Liu R, et al. Rescue of maternal immune activation-induced behavioral abnormalities in adult mouse offspring by pathogen-activated maternal T(reg) cells. Nat Neurosci. 2021;24:818–30.
Mariscal P, Bravo L, Llorca-Torralba M, Razquin J, Miguelez C, Suarez-Pereira I, et al. Sexual differences in locus coeruleus neurons and related behavior in C57BL/6J mice. Biol Sex Differ. 2023;14:64.
Mulvey B, Bhatti DL, Gyawali S, Lake AM, Kriaucionis S, Ford CP, et al. Molecular and functional sex differences of noradrenergic neurons in the mouse locus coeruleus. Cell Rep. 2018;23:2225–35.
Guo X, Li J, Qi Y, Chen J, Jiang M, Zhu L, et al. Telomere length and micronuclei trajectories in APP/PS1 mouse model of Alzheimer’s disease: correlating with cognitive impairment and brain amyloidosis in a sexually dimorphic manner. Aging Cell. 2024;23:e14121.
Kim KH, Cho Y, Lee J, Jeong H, Lee Y, Kim SI, et al. Sexually dimorphic leanness and hypermobility in p16(Ink4a)/CDKN2A-deficient mice coincides with phenotypic changes in the cerebellum. Sci Rep. 2019;9:11167.
Jacobs HIL, Hopkins DA, Mayrhofer HC, Bruner E, van Leeuwen FW, Raaijmakers W, et al. The cerebellum in Alzheimer’s disease: evaluating its role in cognitive decline. Brain. 2018;141:37–47.
Vacher CM, Lacaille H, O’Reilly JJ, Salzbank J, Bakalar D, Sebaoui S, et al. Placental endocrine function shapes cerebellar development and social behavior. Nat Neurosci. 2021;24:1392–401.
Eliot L, Ahmed A, Khan H, Patel J. Dump the “dimorphism”: comprehensive synthesis of human brain studies reveals few male-female differences beyond size. Neurosci Biobehav Rev. 2021;125:667–97.
McCarthy MM, Wright CL. Convergence of sex differences and the neuroimmune system in autism spectrum disorder. Biol Psychiatry. 2017;81:402–10.
Aja E, Jacobs JP. Mommy’s microbes: gestational diabetes mellitus shapes the maternal and infant gut microbiome. Cell Host Microbe. 2024;32:1048–49.
Brown K, Thomson CA, Wacker S, Drikic M, Groves R, Fan V, et al. Microbiota alters the metabolome in an age- and sex- dependent manner in mice. Nat Commun. 2023;14:1348.
Jasarevic E, Howard CD, Morrison K, Misic A, Weinkopff T, Scott P, et al. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat Neurosci. 2018;21:1061–71.
Lipner EM, Murphy SK, Ellman LM. Prenatal maternal stress and the cascade of risk to schizophrenia spectrum disorders in offspring. Curr Psychiatry Rep. 2019;21:99.
Rogers A, Obst S, Teague SJ, Rossen L, Spry EA, Macdonald JA, et al. Association between maternal perinatal depression and anxiety and child and adolescent development: a meta-analysis. JAMA Pediatr. 2020;174:1082–92.
Rosenqvist MA, Sjolander A, Ystrom E, Larsson H, Reichborn-Kjennerud T. Adverse family life events during pregnancy and ADHD symptoms in five-year-old offspring. J Child Psychol Psychiatry. 2019;60:665–75.
Tirumalaraju V, Suchting R, Evans J, Goetzl L, Refuerzo J, Neumann A, et al. Risk of depression in the adolescent and adult offspring of mothers with perinatal depression: a systematic review and meta-analysis. JAMA Netw Open. 2020;3:e208783.
Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota and neurodevelopmental windows: implications for brain disorders. Trends Mol Med. 2014;20:509–18.
Rosario SR, Long MD, Chilakapati S, Gomez EC, Battaglia S, Singh PK, et al. Integrative multi-omics analysis uncovers tumor-immune-gut axis influencing immunotherapy outcomes in ovarian cancer. Nat Commun. 2024;15:10609.
Acknowledgements
This study was supported by Shenzhen Science and Technology Program (KCXFZ20211020163549011, JCYJ20250604183013017 to XAL); the “Nutrition and Care of Maternal & Child Research Fund Project” of Biostime Institute of Nutrition & Care (2020BINCMCF058 to XAL); the STI2030-Major Projects (2022ZD0207100 to ZXC); National Natural Science Foundation of China (NSFC) (32371213 to XAL, 32000710 to ZXC, U20A2016 to ZXC); the Shenzhen Medical Research Funds (D2301002 to XAL); the Outstanding Talents Training Fund in Shenzhen (to LPW); Beijing Life Science Academy (BLSA, 2024100CD0010 to ZXC); Shenzhen Key Basic Research Project (JCYJ20200109115641762 to ZXC), the Guangdong Basic and Applied Basic Research Foundation (2023A1515011743 to XAL); the Technology and Innovation Commission of Shenzhen (ZDSYS20190902093601675 to ZXC).
Author information
Authors and Affiliations
Contributions
XJ analyzed the data, visualized the figures, and drafted the original manuscript; XJ, ZX, QY, and YJ contributed to conducted experiments, data analysis, figures visualization and manuscript formatting; RW participated in the chronic unpredictable mild stress and behavioral experiments; YL and RZ participated in human sample information collection and neonatal faecal sampling; QY and JW performed the electrophysiological recording experiments; XL and ZC conceptualized the project framework and supervised the experimental execution; LW provided essential resources and analytic platforms and assisted with experimental execution; XL and ZC revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jing, X., Xu, Z., Yang, Q. et al. Unraveling sexually dimorphic offspring behaviors: maternal premating stress and the neuro-microbial-metabolic network. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03378-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03378-2