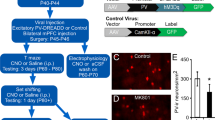
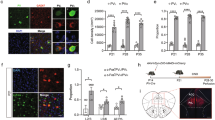
Abstract
To achieve goals in a dynamic environment, the prefrontal cortex (PFC) facilitates various cognitive functions that allow an organism to detect and discriminate information, identify changes in context, and apply this information to representations of known rules and internal states. A failure of organized communication in the PFC is thought to contribute to the emergence of cognitive impairments in psychiatric diseases, with attentional deficits occurring as a fundamental symptom across various conditions. The 22q11.2 microdeletion syndrome is a rare genetic condition that confers a high risk for developing psychiatric and neurodevelopmental disorders, and mouse models have been shown to display attention impairments and PFC pathology that are relevant to clinical populations. Abnormalities in prefrontal parvalbumin-expressing neurons (PVNs) are part of the observed pathophysiology, and studies in rodents have shown that the direct manipulation of these cells can induce behavioral deficits that align with the cognitive symptoms seen in psychiatric diseases. PVNs are a subclass of GABAergic inhibitory cells that facilitate high frequency oscillations that are associated with information processing in the cortex. Because PVNs have such a pivotal role in organizing the input of information to the PFC, their dysfunction could contribute to the manifestation of a variety of cognitive and behavior impairments in disease states. In the present study, we expanded on the role of PVNs in supporting cognition by investigating their involvement in multiple components of attentional functions using a translationally relevant task of focused visual attention, in both non-pathological mice and a model of the 22q11.2 microdeletion syndrome. Using genetically-encoded calcium sensors, fiber photometry, and optogenetics, we describe a novel assessment of how PVN activity is modulated by learning and acute changes in task demands. Interestingly, we observed that task-evoked prefrontal PVN activity was reduced in mice that exhibited poorer attention and in 22q11.2 mutant mice. While PVN activity was shaped across learning in non-pathological mice, mutant mice exhibited a lack of signal dynamics that coincided with attentional deficits. Importantly, we observed that task performance in both poor performing wild-types and 22q11.2 mutants could be alleviated by high frequency (gamma band range) PVN stimulation. Thus, PVNs appear to be involved in the acquisition of task rules and execution of attention and continue to be a promising therapeutic target for cognitive dysfunction in disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
Data is available upon request. Additionally, the datasets generated and analysed in this study will be freely accessible in the Mousebytes repository, https://mousebytes.ca/home.
References
Chamberlin LA, Yang S-S, McEachern EP, Lucas JT, McLeod Ii OW, Rolland CA, et al. Pharmacogenetic activation of parvalbumin interneurons in the prefrontal cortex rescues cognitive deficits induced by adolescent MK801 administration. Neuropsychopharmacology. 2023;48:1267–76.
Kamigaki T, Dan Y. Delay activity of specific prefrontal interneuron subtypes modulates memory-guided behavior. Nat Neurosci. 2017;20:854–63.
Murray AJ, Woloszynowska-Fraser MU, Ansel-Bollepalli L, Cole KL, Foggetti A, Crouch B, et al. Parvalbumin-positive interneurons of the prefrontal cortex support working memory and cognitive flexibility. Sci Rep. 2015;5:16778.
Nguyen R, Venkatesan S, Binko M, Bang JY, Cajanding JD, Briggs C, et al. Cholecystokinin-expressing interneurons of the medial prefrontal cortex mediate working memory retrieval. J Neurosci. 2020;40:2314–31.
Toader O, von Heimendahl M, Schuelert N, Nissen W, Rosenbrock H. Suppression of parvalbumin interneuron activity in the prefrontal cortex recapitulates features of impaired excitatory/inhibitory balance and sensory processing in schizophrenia. Schizophr Bull. 2020;46:981–9.
Cho KK, Davidson TJ, Bouvier G, Marshall JD, Schnitzer MJ, Sohal VS. Cross-hemispheric gamma synchrony between prefrontal parvalbumin interneurons supports behavioral adaptation during rule shift learning. Nat Neurosci. 2020;23:892–902.
Cho KK, Hoch R, Lee AT, Patel T, Rubenstein JL, Sohal VS. Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6+/− mice. Neuron. 2015;85:1332–43.
Cho KK, Shi J, Phensy AJ, Turner ML, Sohal VS. Long-range inhibition synchronizes and updates prefrontal task activity. Nature. 2023;617:548–54.
Patrono E, Hrůzova K, Svoboda J, Stuchlík A. The role of optogenetic stimulations of parvalbumin-positive interneurons in the prefrontal cortex and the ventral hippocampus on an acute MK-801 model of schizophrenia-like cognitive inflexibility. Schizophr Res. 2023;252:198–205.
Ferguson B, Glick C, Huguenard JR. Prefrontal PV interneurons facilitate attention and are linked to attentional dysfunction in a mouse model of absence epilepsy. eLife. 2023;12:e78349.
Kim H, Ährlund-Richter S, Wang X, Deisseroth K, Carlén M. Prefrontal parvalbumin neurons in control of attention. Cell. 2016;164:208–18.
Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702.
Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–7.
Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324:1207–10.
Buzsáki G, Wang X-J. Mechanisms of gamma oscillations. Annu Rev Neurosci. 2012;35:203–25.
Tamura M, Spellman TJ, Rosen AM, Gogos JA, Gordon JA. Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nat Commun. 2017;8:2182.
Karlsson MP, Tervo DG, Karpova AY. Network resets in medial prefrontal cortex mark the onset of behavioral uncertainty. Science. 2012;338:135–9.
Marton TF, Seifikar H, Luongo FJ, Lee AT, Sohal VS. Roles of prefrontal cortex and mediodorsal thalamus in task engagement and behavioral flexibility. J Neurosci. 2018;38:2569–78.
Senkowski D, Gallinat J. Dysfunctional prefrontal gamma-band oscillations reflect working memory and other cognitive deficits in schizophrenia. Biol Psychiatry. 2015;77:1010–9.
Toker L, Mancarci BO, Tripathy S, Pavlidis P. Transcriptomic evidence for alterations in astrocytes and parvalbumin interneurons in subjects with bipolar disorder and schizophrenia. Biol Psychiatry. 2018;84:787–96.
Uhlhaas PJ, Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nat Rev Neurosci. 2010;11:100–13.
Chung DW, Fish KN, Lewis DA. Pathological basis for deficient excitatory drive to cortical parvalbumin interneurons in schizophrenia. Am J Psychiatry. 2016;173:1131–9.
Dienel SJ, Fish KN, Lewis DA. The nature of prefrontal cortical GABA neuron alterations in schizophrenia: markedly lower somatostatin and parvalbumin gene expression without missing neurons. Am J Psychiatry. 2023;180:495–507.
Enwright III JF, Huo Z, Arion D, Corradi JP, Tseng G, Lewis DA. Transcriptome alterations of prefrontal cortical parvalbumin neurons in schizophrenia. Mol Psychiatry. 2018;23:1606–13.
Enwright JF, Sanapala S, Foglio A, Berry R, Fish KN, Lewis DA. Reduced labeling of parvalbumin neurons and perineuronal nets in the dorsolateral prefrontal cortex of subjects with schizophrenia. Neuropsychopharmacology. 2016;41:2206–14.
Glausier J, Fish K, Lewis D. Altered parvalbumin basket cell inputs in the dorsolateral prefrontal cortex of schizophrenia subjects. Mol Psychiatry. 2014;19:30–36.
Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biol Psychiatry. 2015;77:1031–40.
Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–55.
Haenschel C, Bittner RA, Waltz J, Haertling F, Wibral M, Singer W, et al. Cortical oscillatory activity is critical for working memory as revealed by deficits in early-onset schizophrenia. J Neurosci. 2009;29:9481–9.
Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–26.
Hoftman GD, Datta D, Lewis DA. Layer 3 excitatory and inhibitory circuitry in the prefrontal cortex: developmental trajectories and alterations in schizophrenia. Biol Psychiatry. 2017;81:862–73.
Beneyto M, Lewis DA. Insights into the neurodevelopmental origin of schizophrenia from postmortem studies of prefrontal cortical circuitry. Int J Dev Neurosci. 2011;29:295–304.
Kaar SJ, Angelescu I, Marques TR, Howes OD. Pre-frontal parvalbumin interneurons in schizophrenia: a meta-analysis of post-mortem studies. J Neural Transm. 2019;126:1637–51.
Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–22.
Lewis DA, Curley AA, Glausier JR, Volk DW. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012;35:57–67.
Satoh J, Tabira T, Sano M, Nakayama H, Tateishi J. Parvalbumin-immunoreactive neurons in the human central nervous system are decreased in Alzheimer’s disease. Acta Neuropathol. 1991;81:388–95.
Verret L, Mann EO, Hang GB, Barth AM, Cobos I, Ho K, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149:708–21.
Arai H, Emson P, Mountjoy C, Carassco L, Heizmann C. Loss of parvalbumin-immunoreactive neurones from cortex in Alzheimer-type dementia. Brain Res. 1987;418:164–9.
Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature. 2016;540:230–5.
Mikkonen M, Alafuzoff I, Tapiola T, Soininen H, Miettinen R. Subfield-and layer-specific changes in parvalbumin, calretinin and calbindin-D28K immunoreactivity in the entorhinal cortex in Alzheimer’s disease. Neuroscience. 1999;92:515–32.
Lanoue AC, Blatt GJ, Soghomonian J-J. Decreased parvalbumin mRNA expression in dorsolateral prefrontal cortex in Parkinson′ s disease. Brain Res. 2013;1531:37–47.
Lilascharoen V, Wang EH-J, Do N, Pate SC, Tran AN, Yoon CD, et al. Divergent pallidal pathways underlying distinct Parkinsonian behavioral deficits. Nat Neurosci. 2021;24:504–15.
Kim, Thu DC, Tippett LJ, Oorschot DE, Hogg VM, Roxburgh R, et al. Cortical interneuron loss and symptom heterogeneity in Huntington disease. Ann Neurol. 2014;75:717–27.
Reiner A, Shelby E, Wang H, DeMarch Z, Deng Y, Guley NH, et al. Striatal parvalbuminergic neurons are lost in Huntington’s disease: implications for dystonia. Mov Disord. 2013;28:1691–9.
Shaikh TH, Kurahashi H, Saitta SC, O’Hare AM, Hu P, Roe BA, et al. Chromosome 22-specific low copy repeats and the 22q11. 2 deletion syndrome: genomic organization and deletion endpoint analysis. Hum Mol Genet. 2000;9:489–501.
Edelmann L, Pandita RK, Morrow BE. Low-copy repeats mediate the common 3-Mb deletion in patients with velo-cardio-facial syndrome. Am J Hum Genet. 1999;64:1076–86.
Karayiorgou, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci. 1995;92:7612–6.
Maynard T, Haskell G, Peters A, Sikich L, Lieberman J, LaMantia A-S. A comprehensive analysis of 22q11 gene expression in the developing and adult brain. Proc Natl Acad Sci. 2003;100:14433–8.
Schneider M, Debbané M, Bassett AS, Chow EW, Fung WL, van den Bree M, et al. Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 2014;171:627–39. https://doi.org/10.1176/appi.ajp.2013.13070864
Karayiorgou M, Simon TJ, Gogos JA. 22q11. 2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11:402–16.
McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, et al. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41:1223–7. https://doi.org/10.1038/ng.474
Simon TJ, Bish JP, Bearden CE, Ding L, Ferrante S, Nguyen V, et al. A multilevel analysis of cognitive dysfunction and psychopathology associated with chromosome 22q11. 2 deletion syndrome in children. Dev Psychopathol. 2005;17:753–84.
Sobin C, Kiley-Brabeck K, Daniels S, Blundell M, Anyane-Yeboa K, Karayiorgou M. Networks of attention in children with the 22q11 deletion syndrome. Dev Neuropsychol. 2004;26:611–26.
Stoddard J, Beckett L, Simon TJ. Atypical development of the executive attention network in children with chromosome 22q11. 2 deletion syndrome. J Neurodev Disord. 2011;3:76–85.
Bish JP, Ferrante SM, McDonald‐McGinn D, Zackai E, Simon TJ. Maladaptive conflict monitoring as evidence for executive dysfunction in children with chromosome 22q11. 2 deletion syndrome. Dev Sci. 2005;8:36–43.
Larsen KM, Pellegrino G, Birknow MR, Kjær TN, Baaré WFC, Didriksen M, et al. 22q11. 2 deletion syndrome is associated with impaired auditory steady-state gamma response. Schizophr Bull. 2018;44:388–97.
Mancini V, Rochas V, Seeber M, Grent T, Rihs TA, Latrèche C, et al. Oscillatory neural signatures of visual perception across developmental stages in individuals with 22q11. 2 deletion syndrome. Biol Psychiatry. 2022;92:407–18.
Mancini V, Rochas V, Seeber M, Roehri N, Rihs TA, Ferat V, et al. Aberrant developmental patterns of gamma-band response and long-range communication disruption in youths with 22q11. 2 deletion syndrome. Am J Psychiatry. 2022;179:204–15.
Al-Absi A-R, Thambiappa SK, Khan AR, Glerup S, Sanchez C, Landau AM, et al. Df (h22q11)/+ mouse model exhibits reduced binding levels of GABAA receptors and structural and functional dysregulation in the inhibitory and excitatory networks of hippocampus. Mol Cell Neurosci. 2022;122:103769.
Tripathi A, Spedding M, Schenker E, Didriksen M, Cressant A, Jay TM. Cognition-and circuit-based dysfunction in a mouse model of 22q11. 2 microdeletion syndrome: effects of stress. Transl Psychiatry. 2020;10:41.
Nilsson SR, Fejgin K, Gastambide F, Vogt MA, Kent BA, Nielsen V, et al. Assessing the cognitive translational potential of a mouse model of the 22q11. 2 microdeletion syndrome. Cereb Cortex. 2016;26:3991–4003.
Nilsson SR, Heath CJ, Takillah S, Didienne S, Fejgin K, Nielsen V, et al. Continuous performance test impairment in a 22q11. 2 microdeletion mouse model: improvement by amphetamine. Transl Psychiatry. 2018;8:247.
Kim, Hvoslef-Eide M, Nilsson SR, Johnson MR, Herbert BR, Robbins TW, et al. The continuous performance test (rCPT) for mice: a novel operant touchscreen test of attentional function. Psychopharmacology (Berl). 2015;232:3947–66. https://doi.org/10.1007/s00213-015-4081-0
Mar AC, Nilsson SR, Gamallo-Lana B, Lei M, Dourado T, Alsiö J, et al. MAM-E17 rat model impairments on a novel continuous performance task: effects of potential cognitive enhancing drugs. Psychopharmacology (Berl). 2017;234:2837–57.
Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20:31–46.
Hooper SR, Curtiss K, Schoch K, Keshavan MS, Allen A, Shashi V. A longitudinal examination of the psychoeducational, neurocognitive, and psychiatric functioning in children with 22q11. 2 deletion syndrome. Res Dev Disabil. 2013;34:1758–69.
Lewandowski KE, Shashi V, Berry PM, Kwapil TR. Schizophrenic‐like neurocognitive deficits in children and adolescents with 22q11 deletion syndrome. Am J Med Genet Part B: Neuropsychiatr Genet. 2007;144:27–36.
Nestor PG, Faux SF, McCarley RW, Shenton ME, Sands SF. Measurement of visual sustained attention in schizophrenia using signal detection analysis and a newly developed computerized CPT task. Schizophr Res. 1990;3:329–32. https://doi.org/10.1016/0920-9964(90)90018-3
Nuechterlein, KH Vigilance in schizophrenia and related disorders. 1991
Forwood SE, Cowell RA, Bussey TJ, Saksida LM. Multiple cognitive abilities from a single cortical algorithm. J Cogn Neurosci. 2012;24:1807–25.
Gaffan D. Against memory systems. Philos Trans R Soc London. Series B: Biol Sci. 2002;357:1111–21.
Bussey TJ, Saksida LM. Object memory and perception in the medial temporal lobe: an alternative approach. Curr Opin Neurobiol. 2005;15:730–7.
Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. https://doi.org/10.1146/annurev.neuro.24.1.167
Panichello MF, Buschman TJ. Shared mechanisms underlie the control of working memory and attention. Nature. 2021;592:601–5.
Didriksen M, Fejgin K, Nilsson SR, Birknow MR, Grayton HM, Larsen PH, et al. Persistent gating deficit and increased sensitivity to NMDA receptor antagonism after puberty in a new mouse model of the human 22q11. 2 microdeletion syndrome: a study in male mice. J Psychiatry Neurosci. 2017;42:48–58.
Madisen L, Mao T, Koch H, Zhuo J-m, Berenyi A, Fujisawa S, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802.
Dana H, Sun Y, Mohar B, Hulse BK, Kerlin AM, Hasseman JP, et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat Methods. 2019;16:649–57.
Rajendran PS, Challis RC, Fowlkes CC, Hanna P, Tompkins JD, Jordan MC, et al. Identification of peripheral neural circuits that regulate heart rate using optogenetic and viral vector strategies. Nat Commun. 2019;10:1944.
Horner AE, Heath CJ, Hvoslef-Eide M, Kent BA, Kim CH, Nilsson SRO, et al. The touchscreen operant platform for testing learning and memory in rats and mice. Nat Protoc. 2013;8:1961–84. https://doi.org/10.1038/nprot.2013.122
Oomen CA, Hvoslef-Eide M, Heath CJ, Mar AC, Horner AE, Bussey TJ, et al. The touchscreen operant platform for testing working memory and pattern separation in rats and mice. Nat Protoc. 2013;8:2006–21. https://doi.org/10.1038/nprot.2013.124
Frey PW, Colliver JA. Sensitivity and responsivity measures for discrimination learning. Learn Motiv. 1973;4:327–42.
Green, DM, & Swets, JA Signal detection theory and psychophysics (Vol. 1): New York: Wiley 1966.
Skirzewski M, Princz-Lebel O, German-Castelan L, Crooks AM, Kim GK, Tarnow SH, et al. Continuous cholinergic-dopaminergic updating in the nucleus accumbens underlies approaches to reward-predicting cues. Nat Commun. 2022;13:7924.
Oostenveld R, Fries P, Maris E, Schoffelen J-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011:156869.
Weisz N, Dohrmann K, Elbert T. The relevance of spontaneous activity for the coding of the tinnitus sensation. Prog Brain Res. 2007;166:61–70.
Weisz N, Moratti S, Meinzer M, Dohrmann K, Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005;2:e153.
Wieczerzak KB, Patel SV, MacNeil H, Scott KE, Schormans AL, Hayes SH, et al. Differential plasticity in auditory and prefrontal cortices, and cognitive-behavioral deficits following noise-induced hearing loss. Neuroscience. 2021;455:1–18.
Hayes SH, Schormans AL, Sigel G, Beh K, Herrmann B, Allman BL. Uncovering the contribution of enhanced central gain and altered cortical oscillations to tinnitus generation. Prog Neurobiol. 2021;196:101893.
Mukherjee A, Carvalho F, Eliez S, Caroni P. Long-lasting rescue of network and cognitive dysfunction in a genetic schizophrenia model. Cell. 2019;178:1387–1402. e1314.
Norman KJ, Koike H, McCraney SE, Garkun Y, Bateh J, Falk EN, et al. Chemogenetic suppression of anterior cingulate cortical neurons projecting to the visual cortex disrupts attentional behavior in mice. Neuropsychopharmacol Rep. 2021;41:207–14.
Hvoslef-Eide, M, Nilsson, SR, Hailwood, JM, Robbins, TW, Saksida, LM, Mar, AC, et al. Effects of anterior cingulate cortex lesions on a continuous performance task for mice. Brain Neurosci Adv 2018, 2. https://doi.org/10.1177/2398212818772962
Insel N, Barnes CA. Differential activation of fast-spiking and regular-firing neuron populations during movement and reward in the dorsal medial frontal cortex. Cereb Cortex. 2015;25:2631–47.
Jeong H, Kim D, Song M, Paik S-B, Jung MW. Distinct roles of parvalbumin-and somatostatin-expressing neurons in flexible representation of task variables in the prefrontal cortex. Prog Neurobiol. 2020;187:101773.
Kim D, Jeong H, Lee J, Ghim J-W, Her ES, Lee S-H, et al. Distinct roles of parvalbumin-and somatostatin-expressing interneurons in working memory. Neuron. 2016;92:902–15.
Pinto L, Dan Y. Cell-type-specific activity in prefrontal cortex during goal-directed behavior. Neuron. 2015;87:437–50.
Caballero-Puntiverio, Lerdrup L, Grupe M, Larsen C, Dietz A, Andreasen J. Effect of ADHD medication in male C57BL/6J mice performing the rodent Continuous Performance Test. Psychopharmacology (Berl). 2019;236:1839–51.
Robinson ES. Blockade of noradrenaline re-uptake sites improves accuracy and impulse control in rats performing a five-choice serial reaction time tasks. Psychopharmacology (Berl). 2012;219:303–12.
Tomlinson A, Grayson B, Marsh S, Harte MK, Barnes SA, Marshall KM, et al. Pay attention to impulsivity: modelling low attentive and high impulsive subtypes of adult ADHD in the 5-choice continuous performance task (5C-CPT) in female rats. Eur Neuropsychopharmacol. 2014;24:1371–80. https://doi.org/10.1016/j.euroneuro.2014.04.008
Caballero-Puntiverio M, Fitzpatrick CM, Woldbye DP, Andreasen JT. Effects of amphetamine and methylphenidate on attentional performance and impulsivity in the mouse 5-Choice Serial Reaction Time Task. J Psychopharmacol. 2017;31:272–83.
Snitz BE, MacDonald III AW, Carter CS Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes 2006.
Liu SK, Chiu C-H, Chang C-J, Hwang T-J, Hwu H-G, Chen WJ. Deficits in sustained attention in schizophrenia and affective disorders: stable versus state-dependent markers. Am J Psychiatry. 2002;159:975–82.
Filice F, Vörckel KJ, Sungur AÖ, Wöhr M, Schwaller B. Reduction in parvalbumin expression not loss of the parvalbumin-expressing GABA interneuron subpopulation in genetic parvalbumin and shank mouse models of autism. Mol Brain. 2016;9:1–17.
Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–407.
Hussar CR, Pasternak T. Flexibility of sensory representations in prefrontal cortex depends on cell type. Neuron. 2009;64:730–43.
Mansouri FA, Matsumoto K, Tanaka K. Prefrontal cell activities related to monkeys’ success and failure in adapting to rule changes in a Wisconsin Card Sorting Test analog. J Neurosci. 2006;26:2745–56.
Parthasarathy A, Herikstad R, Bong JH, Medina FS, Libedinsky C, Yen S-C. Mixed selectivity morphs population codes in prefrontal cortex. Nat Neurosci. 2017;20:1770–9.
Rigotti M, Barak O, Warden MR, Wang X-J, Daw ND, Miller EK, et al. The importance of mixed selectivity in complex cognitive tasks. Nature. 2013;497:585–90.
Wilent WB, Contreras D. Dynamics of excitation and inhibition underlying stimulus selectivity in rat somatosensory cortex. Nat Neurosci. 2005;8:1364–70.
Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–9.
Roach JP, Churchland AK, Engel TA. Choice selective inhibition drives stability and competition in decision circuits. Nat Commun. 2023;14:147.
Lee S-H, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, et al. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–83.
Hallock HL, Adiraju SS, Miranda-Barrientos J, McInerney JM, Oh S, DeBrosse AC, et al. Electrophysiological correlates of attention in the locus coeruleus–prelimbic cortex circuit during the rodent continuous performance test. Neuropsychopharmacology. 2024;49:521–31.
Cope ZA, Young JW. The five‐choice continuous performance task (5C‐CPT): a cross‐species relevant paradigm for assessment of vigilance and response inhibition in rodents. Curr Protoc Neurosci. 2017;78:9.56. 51–59.56. 18.
Reinert S, Hübener M, Bonhoeffer T, Goltstein PM. Mouse prefrontal cortex represents learned rules for categorization. Nature. 2021;593:411–7.
Xu Y, Wang M-L, Tao H, Geng C, Guo F, Hu B, et al. ErbB4 in parvalbumin-positive interneurons mediates proactive interference in olfactory associative reversal learning. Neuropsychopharmacology. 2022;47:1292–303.
Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96.
Sparta DR, Hovelsø N, Mason AO, Kantak PA, Ung RL, Decot HK, et al. Activation of prefrontal cortical parvalbumin interneurons facilitates extinction of reward-seeking behavior. J Neurosci. 2014;34:3699–705.
Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–50.
Gregoriou GG, Paneri S, Sapountzis P. Oscillatory synchrony as a mechanism of attentional processing. Brain Res. 2015;1626:165–82.
Santos-Silva T, dos Santos Fabris D, de Oliveira CL, Guimarães FS, Gomes FV. Prefrontal and hippocampal parvalbumin interneurons in animal models for schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2024;50:210–23.
Terstege DJ, Epp JR. Parvalbumin as a sex-specific target in Alzheimer’s disease research, A mini-review. Neurosci Biobehav Rev. 2023;153:105370.
Hijazi S, Smit AB, van Kesteren RE. Fast-spiking parvalbumin-positive interneurons in brain physiology and Alzheimer’s disease. Mol Psychiatry. 2023;28:4954–67.
Fung SJ, Fillman SG, Webster MJ, Weickert CS. Schizophrenia and bipolar disorder show both common and distinct changes in cortical interneuron markers. Schizophr Res. 2014;155:26–30.
Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci. 2000;97:13372–7.
Wöhr M, Orduz D, Gregory P, Moreno H, Khan U, Vörckel KJ, et al. Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl Psychiatry. 2015;5:e525–e525.
Orduz D, Bischop DP, Schwaller B, Schiffmann SN, Gall D. Parvalbumin tunes spike‐timing and efferent short‐term plasticity in striatal fast spiking interneurons. J Physiol. 2013;591:3215–32.
Demeter E, Guthrie SK, Taylor SF, Sarter M, Lustig C. Increased distractor vulnerability but preserved vigilance in patients with schizophrenia: evidence from a translational Sustained Attention Task. Schizophr Res. 2013;144:136–41. https://doi.org/10.1016/j.schres.2013.01.003
Cao W, Lin S, Xia Q-q, Du Y-l, Yang Q, Zhang M-y, et al. Gamma oscillation dysfunction in mPFC leads to social deficits in neuroligin 3 R451C knockin mice. Neuron. 2018;97:1253–1260. e1257.
Acknowledgements
This research was supported by through the Canada First Research Excellence Fund (CFREF), Brain Canada, the Canadian Institutes of Health Research (CHIR PJT 426966), the Natural Sciences and Engineering Research Council (NSERC RGPIN-2019-06102 RGPIN-2019-06087), the Canada Foundation for Innovation, and the Ontario Research Fund. All figures were created in BioRender. Dexter, T. (2026) https://BioRender.com/csljh96. LMS is the Canada Research Chair in Translational Cognitive Neuroscience and TJB is the Western Research Chair in Behavioural Neuroscience.
Author information
Authors and Affiliations
Contributions
The project was conceived by T.D.D, T.J.B, L.M.S., and D.P. and supervised by D.P., T.J.B. and L.M.S. T.D.D performed the behavioral experiments, analyzed the data, generated the figures, and wrote the manuscript. T.D.D., T.J.B, and L.M.S edited the manuscript. A.L.S. conducted the electrophysiology experiments, analyzed the data, generated Fig. 4, and wrote the electrophysiology results and methods sections. D.P. wrote custom photometry and optogenetic analysis scripts and provided training and supervision. S.H. assisted in testing mice included in Supplementary Figure 5. T.D.D. and M.M.F.M. processed the brain tissue for histology. M.M.F.M. performed the immunohistochemistry and generated the images. B.L.A. provided insight throughout the project and supervised the electrophysiology experiments.
Corresponding author
Ethics declarations
Competing interests
T.J.B. and L.M.S. have established a series of targeted cognitive tests for animals, administered via touchscreen within a custom environment known as the “Bussey-Saksida touchscreen chamber”. Cambridge Enterprise, the technology transfer office of the University of Cambridge, supported commercialisation of the Bussey-Saksida chamber, culminating in a license to Campden Instruments. Any financial compensation received from commercialisation of the technology is fully invested in further touchscreen development and/or maintenance. T.D., D.P., A.L.S., S.H., M.M.F.M., B.L.A. declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dexter, T.D., Palmer, D., Schormans, A.L. et al. Prefrontal parvalbumin neurons as a target for enhancing cognition in non-pathological and 22q11.2 microdeletion syndrome mice. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03386-2
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03386-2