Abstract
Mosaic chromosomal alterations (mCAs) accumulate in the brain tissues and are associated with psychiatric disorders. The association between mCAs in circulating blood and schizophrenia (SCZ) and bipolar disorders (BD) has not been fully evaluated. We detected mCAs from blood samples in 2470 SCZ, 3732 BD, and 177,773 control subjects. The associations between mCAs and SCZ or BD were evaluated using age-adjusted logistic regression models, further evaluated in age subgroups. We analyzed the associations between high cell fraction (CF) mosaic events (CF-mCAs >5% or CF-mCAs >10%) and SCZ or BD in the same way. Furthermore, we assessed the interaction between mCAs and genetic risk scores for SCZ or BD. Autosomal mCAs, especially mosaic loss events, increased in both SCZ and BD (SCZ; OR = 1.78, P = 4.9×10-6, BD; OR = 1.41, P = 0.0025). These associations were highlighted in the young-age subgroup (SCZ; OR = 7.01, P = 1.7×10-16, BD; OR = 4.01, P = 2.9×10-8). In addition, the effect sizes of losses increased in a CF-dependent manner in both SCZ and BD. Loss events with high cell fraction interacted with polygenic risk score in SCZ (P = 0.0098). SCZ or BD were characterized by the presence of a high burden of mosaic losses in blood, especially in young age, suggesting the common somatic pathophysiological mechanisms between these psychiatric diseases. The possible interaction between losses and PRS for SCZ supports the genetic and environmental cross-talk in SCZ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Statistical codes are available from Chikashi Terao (ORCID 0000-0002-6452-4095) at any time, only on reasonable request. The Mosaic Chromosomal Alterations (MoChA) pipelines used for mosaic calling (mocha.wdl) are available at (https://github.com/freeseek/mochawdl). The genotype and IDAT data of BBJ used for this research was available at the website of the NBDC Human Database (https://humandbs.dbcls.jp/en/) of the Database Center for Life Science (DBCLS) / the Joint Support-Center for Data Science Research (DS) of the Research Organization of Information and Systems (ROIS). The dataset ID are JGAD000836 and JGAS000114.
References
Robinson N, Bergen SE. Environmental Risk Factors for Schizophrenia and Bipolar Disorder and Their Relationship to Genetic Risk: Current Knowledge and Future Directions. Front Genet. 2021;12:686666.
Trubetskoy V, Pardinas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
Vijg J, Dong X. Pathogenic Mechanisms of Somatic Mutation and Genome Mosaicism in Aging. Cell. 2020;182:12–23.
Bizzotto S, Walsh CA. Genetic mosaicism in the human brain: from lineage tracing to neuropsychiatric disorders. Nat Rev Neurosci. 2022;23:275–86.
Bae T, Tomasini L, Mariani J, Zhou B, Roychowdhury T, Franjic D, et al. Different mutational rates and mechanisms in human cells at pregastrulation and neurogenesis. Science. 2018;359:550–5.
Lodato MA, Rodin RE, Bohrson CL, Coulter ME, Barton AR, Kwon M, et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science. 2018;359:555–9.
D’Gama AM, Walsh CA. Somatic mosaicism and neurodevelopmental disease. Nat Neurosci. 2018;21:1504–14.
Liu X, Kamatani Y, Terao C. Genetics of autosomal mosaic chromosomal alteration (mCA). J Hum Genet. 2021;66:879–85.
Maury EA, Sherman MA, Genovese G, Gilgenast TG, Kamath T, Burris SJ, et al. Schizophrenia-associated somatic copy-number variants from 12,834 cases reveal recurrent NRXN1 and ABCB11 disruptions. Cell Genom. 2023;3:100356.
Chang K, Jian X, Wu C, Gao C, Li Y, Chen J et al. The contribution of mosaic chromosomal alterations to schizophrenia. Biol Psychiatry 2024;97:198–207.
Maury EA, Jones A, Seplyarskiy V, Nguyen TTL, Rosenbluh C, Bae T, et al. Somatic mosaicism in schizophrenia brains reveals prenatal mutational processes. Science. 2024;386:217–24.
Ju YS, Martincorena I, Gerstung M, Petljak M, Alexandrov LB, Rahbari R, et al. Somatic mutations reveal asymmetric cellular dynamics in the early human embryo. Nature. 2017;543:714–8.
Ikeda M, Aleksic B, Kirov G, Kinoshita Y, Yamanouchi Y, Kitajima T, et al. Copy number variation in schizophrenia in the Japanese population. Biol Psychiatry. 2010;67:283–6.
Ikeda M, Aleksic B, Kinoshita Y, Okochi T, Kawashima K, Kushima I, et al. Genome-wide association study of schizophrenia in a Japanese population. Biol Psychiatry. 2011;69:472–8.
Ikeda M, Takahashi A, Kamatani Y, Okahisa Y, Kunugi H, Mori N, et al. A genome-wide association study identifies two novel susceptibility loci and trans population polygenicity associated with bipolar disorder. Mol Psychiatry. 2018;23:639–47.
Nagai A, Hirata M, Kamatani Y, Muto K, Matsuda K, Kiyohara Y, et al. Overview of the BioBank Japan Project: Study design and profile. J Epidemiol. 2017;27:S2–S8.
Cannon M, Jones P, Huttunen MO, Tanskanen A, Huttunen T, Rabe-Hesketh S, et al. School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry. 1999;56:457–63.
Clemmensen L, Vernal DL, Steinhausen HC. A systematic review of the long-term outcome of early onset schizophrenia. BMC Psychiatry. 2012;12:150.
Maglione JE, Thomas SE, Jeste DV. Late-onset schizophrenia: do recent studies support categorizing LOS as a subtype of schizophrenia? Curr Opin Psychiatry. 2014;27:173–8.
Kennedy KP, Cullen KR, DeYoung CG, Klimes-Dougan B. The genetics of early-onset bipolar disorder: A systematic review. J Affect Disord. 2015;184:1–12.
Arnold I, Dehning J, Grunze A, Hausmann A. Old age bipolar disorder-epidemiology, aetiology and treatment. Medicina (Kaunas). 2021;57:587.
Terao C, Suzuki A, Momozawa Y, Akiyama M, Ishigaki K, Yamamoto K, et al. Chromosomal alterations among age-related haematopoietic clones in Japan. Nature. 2020;584:130–5.
Uchiyama S, Ishikawa Y, Ikari K, Honda S, Hikino K, Tanaka E, et al. Mosaic loss of chromosome Y characterises late-onset rheumatoid arthritis and contrasting associations of polygenic risk score based on age at onset. Ann Rheum Dis. 2025;84:1313–23.
Genomes Project C, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74.
Ito S, Liu X, Ishikawa Y, Conti DD, Otomo N, Kote-Jarai Z, et al. Androgen receptor binding sites enabling genetic prediction of mortality due to prostate cancer in cancer-free subjects. Nat Commun. 2023;14:4863.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75.
Delaneau O, Zagury JF, Robinson MR, Marchini JL, Dermitzakis ET. Accurate, scalable and integrative haplotype estimation. Nat Commun. 2019;10:5436.
Ge T, Chen CY, Ni Y, Feng YA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019;10:1776.
Lam M, Chen CY, Li Z, Martin AR, Bryois J, Ma X, et al. Comparative genetic architectures of schizophrenia in East Asian and European populations. Nat Genet. 2019;51:1670–8.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29.
Higgins-Chen AT, Boks MP, Vinkers CH, Kahn RS, Levine ME. Schizophrenia and epigenetic aging biomarkers: increased mortality, reduced cancer risk, and unique clozapine effects. Biol Psychiatry. 2020;88:224–35.
Okazaki S, Numata S, Otsuka I, Horai T, Kinoshita M, Sora I, et al. Decelerated epigenetic aging associated with mood stabilizers in the blood of patients with bipolar disorder. Transl Psychiatry. 2020;10:129.
Lodato MA, Woodworth MB, Lee S, Evrony GD, Mehta BK, Karger A, et al. Somatic mutation in single human neurons tracks developmental and transcriptional history. Science. 2015;350:94–98.
Sherman MA, Rodin RE, Genovese G, Dias C, Barton AR, Mukamel RE, et al. Large mosaic copy number variations confer autism risk. Nat Neurosci. 2021;24:197–203.
Rodin RE, Dou Y, Kwon M, Sherman MA, D’Gama AM, Doan RN, et al. The landscape of somatic mutation in cerebral cortex of autistic and neurotypical individuals revealed by ultra-deep whole-genome sequencing. Nat Neurosci. 2021;24:176–85.
Hansda AK, Tiwari A, Dixit M. Current status and future prospect of FSHD region gene 1. J Biosci. 2017;42:345–53.
Olfson M, Stroup TS, Huang C, Wall MM, Crystal S, Gerhard T. Suicide risk in medicare patients with schizophrenia across the life span. JAMA Psychiatry. 2021;78:876–85.
Miller JN, Black DW. Bipolar disorder and suicide: a review. Curr Psychiatry Rep. 2020;22:6.
Otsuka I, Uchiyama S, Shirai T, Liu X, Takahashi M, Kamatani Y et al. Increased somatic mosaicism in autosomal and X chromosomes for suicide death. Mol Psychiatry 2025;30:881–8.
Russo P, Prinzi G, Proietti S, Lamonaca P, Frustaci A, Boccia S, et al. Shorter telomere length in schizophrenia: Evidence from a real-world population and meta-analysis of most recent literature. Schizophr Res. 2018;202:37–45.
Acknowledgements
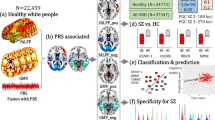
This work was supported by the Japan Agency for Medical Research and Development (AMED) grants 21ek0109555 (C.T.), 21tm0424220 (C.T.), 21ck0106642 (C.T.), 23ek0410114 (C.T.), 23tm0424225 (C.T.), JP21wm0425008 (N.I. and M.I.), JP23tm0524001 (M.I.), JP21wm0525024 (M.I.), 21tm0424220 (M.I.) and JP23dk0307123 (M.I.); the Japan Society for the Promotion of Science (JSPS) KAKENHI grant JP20H00462 (C.T.), JP21H02854 (M.I.), 24K02381 (M.I.) and JP22H03003 (N.I.); and Takeda Hosho Grants for Research in Medicine. The cartoons shown in Fig. 1 were created using Bio-Render. com. We thank the staff of BBJ for collecting and managing the samples and clinical information.
Author information
Authors and Affiliations
Contributions
M.I., N.I. and C.T. conceived the project. S.U. and C.T. analysed the data. G.G. developed MoChA pipelines for mosaic calling. T.S collected the detailed clinical information. S.U., T.S., X.L., Y.I., K.H., and C.T. wrote the manuscript. All authors have critically reviewed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
S.U., T.S., X.L., Y.I., K.H., M.I., N.I. and C.T. have no conflicts of interest. G.G. declared competing interests, and patent application PCT/WO2019/079493 has been filed for the mosaic chromosomal alteration detection method used in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uchiyama, S., Saito, T., Liu, X. et al. Associations between mosaic loss and schizophrenia or bipolar disorder of young age. Mol Psychiatry (2026). https://doi.org/10.1038/s41380-025-03397-z
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03397-z