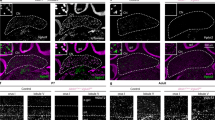
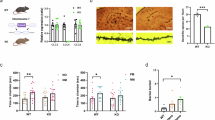
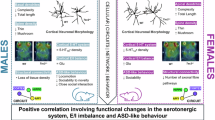
Abstract
Connections between one of the main outputs of the cerebellar cortex, the lateral cerebellar nuclei (LCN), to reward-related circuits may explain social deficits resulting from cerebellar critical period perturbations. To examine the influence of LCN development on social behavior, local neural activity was manipulated in mice using Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) from postnatal day 21–35. Adolescent social behavior was assessed using a three-chamber assay. Results revealed the LCN perturbation abolished male social preference. LCN manipulated mice were found to have less neural activation (cFos) in the ventral tegmental area, nucleus accumbens, and anterior cingulate cortex (ACC). Additionally, mice that received the excitatory DREADD perturbation showed increased dendritic complexity in the ACC. These results suggest a chronic perturbation in the LCN during juvenile and early adolescent development impacts social behavior and results in changes to cortical activity and structure observed in adolescence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The dataset is available at Arizona State University dataverse: https://doi.org/10.48349/ASU/4MT3XU and https://github.com/verpeutlab/Dentatesocial
Code availability
All experimental and analysis code is available at Arizona State University dataverse: https://doi.org/10.48349/ASU/4MT3XU and https://github.com/verpeutlab/Dentatesocial
References
Badura A, Verpeut JL, Metzger JW, Pereira TD, Pisano TJ, Deverett B, et al. Normal cognitive and social development require posterior cerebellar activity. eLife. 2018;7:e36401.
Kelly E, Meng F, Fujita H, Morgado F, Kazemi Y, Rice L, et al. Regulation of autism-relevant behaviors by cerebellar–prefrontal cortical circuits. Nat Neurosci. 2020;23:1102–10.
Kim Y, Umadevi Venkataraju K, Pradhan K, Mende C, Taranda J, Turaga S, et al. Mapping social behavior-induced brain activation at cellular resolution in the mouse. Cell Rep. 2015;10:292–305.
Hur SW, Safaryan K, Yang L, Blair HT, Masmanidis SC, Mathews PJ, et al. Correlated signatures of social behavior in cerebellum and anterior cingulate cortex. eLife. 2024;12:RP88439.
Stoodley CJ, D’Mello AM, Ellegood J, Jakkamsetti V, Liu P, Nebel MB, et al. Altered cerebellar connectivity in autism and cerebellar-mediated rescue of autism-related behaviors in mice. Nat Neurosci. 2017;20:1744–51.
Pisano TJ, Dhanerawala ZM, Kislin M, Bakshinskaya D, Engel EA, Hansen EJ. Homologous orginization of cerebellar pathways to sensory, motor, and associative forebrain. Cell Rep. 2021;36:109721.
Gao Z, Proietti-Onori M, Lin Z, Ruigrok TJH, Hoebeek FE, De Zeeuw CI, et al. Excitatory cerebellar nucleocortical circuit provides internal amplification during associative conditioning. Neuron. 2016;89:645–57.
Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–34.
Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci. 2003;23:8432–44.
Dayan M, Olivito G, Molinari M, Cercignani M, Bozzali M, Loggio M. Impact of cerebellar atrophy on cortical gray matter and cerebellar peduncles as assessed by voxel-based morphometry and high angular resolution diffusion imaging. Funct Neurol. 2016;31:239–48.
Olivito G, Clausi S, Laghi F, Maria Tedesco A, Baiocco R, Mastropasqua C, et al. Resting-state functional connectivity changes between dentate nucleus and cortical social brain regions in autism spectrum disorders. Cerebellum. 2017;16:283–92.
Kana RK, Maximo JO, Williams DL, Keller TA, Schipul SE, Cherkassky VL, et al. Aberrant functioning of the theory-of-mind network in children and adolescents with autism. Mol Autism. 2015;6:59.
Hadland KA, Rushworth MFS, Gaffan D, Passingham RE. The effect of cingulate lesions on social behaviour and emotion. Neuropsychologia. 2003;41:919–31.
Guo B, Chen J, Chen Q, Ren K, Feng D, Mao H, et al. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat Neurosci. 2019;22:1223–34.
Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. J Neurosci. 2005;25:8534–42.
Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta). Behav Neurosci. 2006;120:761–86.
Toyoizumi T, Miyamoto H, Yazaki-Sugiyama Y, Atapour N, Hensch TK, Miller KK. A theory of the transition to critical period plasticity: inhibition selectively suppresses spontaneous activity. Neuron. 2013;80:51–63.
Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705.
Hensch TK, Fagiolini M, Mataga N, Stryker M, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–8.
Ribot J, Breton R, Calvo CF, Moulard J, Ezan P, Zapata J, et al. Astrocytes close the mouse critical period for visual plasticity. Science. 2021;373:77–81.
Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–17.
Wang SS-H, Kloth AD, Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83:518–32.
Gibson JM, Howland CP, Ren C, Howland C, Vernino A, Tsai PT. A critical period for development of cerebellar-mediated autism-relevant social behavior. J Neurosci. 2022;42:2804–23.
D’Mello AM, Stoodley CJ. Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci. 2015;9:408.
Lyle TT, Verpeut JL. Adolescent cerebellar nuclei manipulation alters reversal learning and perineuronal net intensity independently in male and female mice. J Neurosci. 2025;45:e2182232024.
van der Heijden ME, Gill JS, Sillitoe RV. Abnormal cerebellar development in autism spectrum disorders. Dev Neurosci. 2021;43:181–90.
Verpeut JL, Bergeler S, Kislin M, Townes FW, Klibaite U, Dhanerawala ZM, et al. Cerebellar contributions to a brainwide network for flexible behavior in mice. Commun Biol. 2023;6:605.
Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, et al. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–51.
Bariselli, Hörnberg S, Prévost-Solié H, Musardo C, Hatstatt-Burklé S, Scheiffele P, et al. Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nat Commun. 2018;9:3173.
Solié C, Girard B, Righetti B, Tapparel M, Bellone C. VTA dopamine neuron activity encodes social interaction and promotes reinforcement learning through social prediction error. Nat Neurosci. 2022;25:86–97.
Northcutt KV, Nguyen JMK. Female juvenile play elicits Fos expression in dopaminergic neurons of the VTA. Behav Neurosci. 2014;128:178–86.
Peña CJ, DeBerardine M, Sullivan KE. Molecular heterogeneity and development of the ventral tegmental area. Curr Opin Behav Sci. 2025;61:101478.
Novello M, Bosman LWJ, De Zeeuw CI. A systematic review of direct outputs from the cerebellum to the brainstem and diencephalon in mammals. Cerebellum. 2024;23:210–39.
Nio E, Pais Pereira P, Diekmann N, Petrenko M, Doubliez A, Ernst TE, et al. Human cerebellum and ventral tegmental area interact during extinction of learned fear. eLife. 2025;14:RP105399.
Meola A, Comert A, Yeh F-C, Sivakanthan S, Fernandez-Miranda JC. The nondecussating pathway of the dentatorubrothalamic tract in humans: human connectome-based tractographic study and microdissection validation. J Neurosurg. 2016;124:1406–12.
Ikai Y, Takada M, Shinonaga Y, Mizuno N. Dopaminergic and non-dopaminergic neurons in the ventral tegmental area of the rat project, respectively, to the cerebellar cortex and deep cerebellar nuclei. Neuroscience. 1992;51:719–28.
Carta I, Chen CH, Schott AL, Dorizan S, Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science. 2019;363:eaav0581.
Shan Q, Hu Y, Chen S, Tian Y. Nucleus accumbens dichotomically controls social dominance in male mice. Neuropsychopharmacology. 2022;47:776–87.
Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803.
Dreyer JK, Vander Weele CM, Lovic V, Aragona BJ. Functionally distinct dopamine signals in nucleus accumbens core and shell in the freely moving rat. J Neurosci. 2016;36:98–112.
Borland JM. The effects of different types of social interactions on the electrophysiology of neurons in the nucleus accumbens in rodents. Neurosci Biobehav Rev. 2024;164:105809.
Sackett DA, Saddoris MP, Carelli RM. Nucleus accumbens shell dopamine preferentially tracks information related to outcome value of reward. eNeuro. 2017;4:ENEURO.0058–17.2017.
D’Ambra AF, Vlasov K, Jung Jung S, Ganesan S, Antzoulatos EG, Fioravante D. Cerebellar activation bidirectionally regulates nucleus accumbens core and medial shell. eLife. 2023;12:RP87252.
Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns M, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302.
Pereira TD, Tabris N, Matsliah A, Turner DM, Li J, Ravindranath S, et al. SLEAP: A deep learning system for multi-animal pose tracking. Nat Methods. 2022;19:486–95.
Wu R, Jiana X, Wu X, Pang J, Tang Y, Ren Z, et al. Interspecific differences in sociability, social novelty preference, anxiety-and depression-like behaviors between Brandt’s voles and C57BL/6J mice. Behav Processes. 2022.
Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–73.
Lammel S, Kook Lim B, Ran C, Wui Huang K, Betley MJ, Tye KM, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–7.
Kana RK, Libero LE, Moore MS. Disrupted cortical connectivity theory as an explanatory model for autism spectrum disorders. Phys. Life Rev. 2011;8:410–37.
Pisano TJ, Dhanerawala ZM, Kislin M, Bakshinskaya D, Engel EA, Hansen EJ, et al. Homologous organization of cerebellar pathways to sensory, motor, and associative forebrain. Cell Rep. 2021;36:109721.
Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51.
Wilson MD, Sethi S, Lein PJ, Keil KP. Valid statistical approaches for analyzing sholl data: Mixed effects versus simple linear models. J Neurosci Methods. 2017;279:33–43.
Colyn L, Venzala E, Marco S, Perez-Otaño I, Tordera RM. Chronic social defeat stress induces sustained synaptic structural changes in the prefrontal cortex and amygdala. Behav Brain Res. 2019;373:112079.
Lin Y-C, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci. 2010;33:349–78.
Penzes P, Cahill ME, Jones KA, VanLeeuwen J-E, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–93.
Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54.
Gipson CD, Olive MF. Structural and functional plasticity of dendritic spines - root or result of behavior? Genes Brain Behav. 2017;16:101–17.
Chao OY, Marron Fernandez de Velasco E, Saurav Pathak S, Maitra S, Zhang H, Duvick L, et al. Targeting inhibitory cerebellar circuitry to alleviate behavioral deficits in a mouse model for studying idiopathic autism. Neuropsychopharmacology. 2020;45:1159–70.
Edge AL, Marple-Horvat DE, Apps R. Lateral cerebellum: functional localization within crus I and correspondence to cortical zones. Eur J Neurosci. 2003;18:1468–85.
Voogd, J & Bigaré, F Topographical distribution of olivary and cortico nuclear fibers in the cerebellum: A review. in The Inferior Olivary Nucleus (ed. Courville, J) 207-34 (New York: Raven Press, pp. 1980).
Voogd J, Ruigrok TJ. Transverse and longitudinal patterns in the mammalian cerebellum. Prog Brain Res. 1997;114:21–37.
Vogt CC, Zipple MN, Sprockett DD, Miller CH, Hardy SX, Arthur MK, et al. Female behavior drives the formation of distinct social structures in C57BL/6J versus wild-derived outbred mice in field enclosures. BMC Biol. 2024;22:35.
Ramsey LA, Holloman FM, Hope BT, Shaham Y, Venniro M. Waving through the window: A model of volitional social interaction in female mice. Biol Psychiatry. 2022;91:988–97.
Harrison N, Lindholm AK, Dobay A, Halloran O, Manser A, König B. Female nursing partner choice in a population of wild house mice (Mus musculus domesticus). Front Zool. 2018;15:4.
Palanza P, Re L, Mainardi D, Brain PF, Parmigiani S. Male and female competitive strategies of wild house mice pairs (mus Musculus domesticus) confronted with intruders of different sex and age in artificial territories. Behaviour. 1996;133:863–82.
Mackintosh JH. Territory formation by laboratory mice. Anim Behav. 1970;18:177–83.
Rogers TD, Dickson PE, McKimm E, Heck DH, Goldowitz D, Blaha CD, et al. Reorganization of circuits underlying cerebellar modulation of prefrontal cortical dopamine in mouse models of autism spectrum disorder. Cerebellum. 2013;12:547–56.
Apps MAJ, Rushworth MFS, Chang SWC. The anterior cingulate gyrus and social cognition: Tracking the motivation of others. Neuron. 2016;90:692–707.
Kitagawa K, Takemoto T, Seiriki K, Kasai A, Hashimoto H, Nakazawa T. Socially activated neurons in the anterior cingulate cortex are essential for social behavior in mice. Biochem Biophys Res Commun. 2024;726:150251.
Proaño SB, Krentzel AA, Meitzen J. Differential and synergistic roles of 17β-estradiol and progesterone in modulating adult female rat nucleus accumbens core medium spiny neuron electrophysiology. J Neurophysiol. 2020;123:2390–405.
Rodriguez-Echemendia PL, Carelli RM. Sex differences in oscillatory signaling dynamics in the prelimbic cortex and nucleus accumbens core during negative affect. Behav Brain Res. 2025;480:115404.
Liu J, Zubieta J-K, Heitzeg M. Sex differences in anterior cingulate cortex activation during impulse inhibition and behavioral correlates. Psychiatry Res. 2012;201:54–62.
Hidalgo-Lopez E, Noachtar I, Pletzer B. Hormonal contraceptive exposure relates to changes in resting state functional connectivity of anterior cingulate cortex and amygdala. Front Endocrinol (Lausanne). 2023;14:1131995.
Peter S. Dysfunctional cerebellar Purkinje cells contribute to autism-like behavior in Shank3 mutant mice. Nat Neurosci. 2016;19:622–30.
Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15.
Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–42.
Peñagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–46.
Otazu GH, Li Y, Lodato Z, Elnasher A, Keever KM, Li Y, et al. Neurodevelopmental malformations of the cerebellum and neocortex in the Shank3 and Cntnap2 mouse models of autism. Neurosci Lett. 2021;765:136257.
Koekkoek SKE, Yamagushi K, Milojkovic BA, Dortland BR, Ruigrok TJH, Amex R, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–52.
Bruno CA, O’Brien C, Bryant S, Memes JI, Estrin DJ, Pizzano C, et al. pMAT: An open-source software suite for the analysis of fiber photometry data. Pharmacol Biochem Behav. 2021;201:173093.
Simpson EH, Akam T, Patriarchi T, Blanco-Pozo M, Burgeno L, Mohebi A, et al. Lights, fiber, action! A primer on in vivo fiber photometry. Neuron. 2024;112:718–39.
Bridge MF, Wilson LR, Panda S, Stevanovic KD, Letsinger AC, McBride S, et al. FiPhA: an open-source platform for fiber photometry analysis. Neurophotonics. 2024;11:014305.
Acknowledgements
This work was supported by: the Institute for Mental Health Research, Institute for Social Science Research, Nancy Eisenberg Junior Faculty scholar Award, Arizona Department of Health Sciences (ADHS14-052688), US Department of Health and Human Services and the state of Arizona (ADHS Grant No. CTR057001), National Institute on Aging (NIA) of the National Institutes of Health (NIH) (P30AG019610), Arizona Alzheimer’s Disease Research Center REC Fellows Program and Arizona Alzheimer’s Consortium. Figures were created with Biorender.com. We would like to thank Samantha Bowser for animal colony management, Federico Sanabria for statistical analysis discussions, and Mikhail Kislin for assistance with calcium signaling analysis.
Author information
Authors and Affiliations
Contributions
Tristan Lyle: Conceptualization, data analysis and curation, validation, investigation, visualization, methodology, writing, and project administration. Kristin Masho Elbeh: Data analysis, curation, and writing. Daniel Chambers: Data analysis and curation. Henrique Vierira: Data analysis and curation. Jessica L Verpeut: Conceptualization, data analysis and curation, funding acquisition, validation, investigation, visualization, methodology, writing, and project administration.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All study protocols and methods were approved by the Institutional Animal Care and Use Committee at Arizona State University (protocol #24-2052 R) in accordance with guidelines from the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lyle, T.T., Masho Elbeh, K., Chambers, D. et al. Attenuation of social preference and alteration of cortical neurons following adolescent cerebellar nuclei perturbation. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03404-3
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03404-3