Abstract
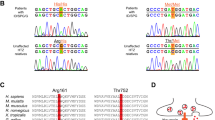
Loss of synaptic upscaling, a post-synaptic homeostatic plasticity, has been reported in mouse models of autism, but the underlying mechanism remains unknown. Glutamate receptor interacting protein 1 (GRIP1) binds AMPA receptor 2 (GluA2) through its PDZ domains 4-6 where gain-of-function variants were described in autism. We characterized mice carrying one variant, GRIP1-I586L (murine I507L), that shows increased binding with GluA2. Grip1-I507L mice exhibit impaired social interaction and increased repetitive behaviors, increased neuronal excitability and excitatory-to-inhibitory ratio in the medial prefrontal cortex. Grip1-I507L cortical neurons show a loss of synaptic upscaling to tetrodotoxin-induced inactivity. Basal phosphorylation of GluA2-Y876 is increased, which is consistent with increased binding to GluA2 while lack of further induction to inactivity contributes to loss of synaptic upscaling. Phosphorylation of GluA2-S880 that regulates Hebbian plasticity is not altered. These results support that gain-of-function GRIP1 variants are a novel cause of autism-related impaired social interaction and increased repetitive behavior and implicate that dysregulated phosphorylation of GluA2-Y876 is a novel mechanism for loss of synaptic upscaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data supporting the key findings of this study is presented in the article and its supplementary information, and available from the corresponding author upon request. Correspondence and requests for materials should be addressed to twang9@jhmi.edu.
References
Jeste S, Geschwind D. Disentangling the heterogeneity of autism spectrum disorder through genetic findings. Nat Rev Neurol. 2014;10:74–81.
Masi A, DeMayo M, Glozier N, Guastella A. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci Bull. 2017;33:183–93.
Hyman S, Levy S, Myers S, COUNCIL ON CHILDREN WITH DISABILITIES, SECTION ON DEVELOPMENTAL AND BEHAVIORAL PEDIATRICS. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145:e20193447.
Turrigiano G, Leslie K, Desai N, Rutherford L, Nelson SB. Activity dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–6.
Shepherd J, Huganir R. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–43.
Turrigiano G. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–35.
Diering G, Huganir R. The AMPA receptor code of synaptic plasticity. Neuron. 2018;100:314–29.
Zhang Z, Marro S, Zhang Y, Arendt K, Patzke C, Zhou B, et al. The fragile X mutation impairs homeostatic plasticity in human neurons by blocking synaptic retinoic-acid signaling. Sci Transl Med. 2018;19:eaar4338.
Blackman M, Djukic D, Nelson S, Turrigiano G. A critical and cell-autonomous role for MeCP2 in synaptic scaling up. J Neurosci. 2012;32:13529–36.
Pastuzyn E, Shepherd J. Activity-dependent arc expression and homeostatic synaptic plasticity are altered in neurons from a mouse model of angelman syndrome. Front Mol Neurosci. 2017;10:234.
Tatavarty V, Pacheco A, Kuhnle C, Lin H, Koundinya P, Miska N, et al. Autism-Associated Shank3 is essential for homeostatic compensation in Rodent V1. Neuron. 2020;106:769–707.
Tan H, Queenan B, Huganir R. GRIP1 is required for homeostatic regulation of AMPAR trafficking. Proc Natl Acad Sci USA. 2015;112:10026–31.
Tan HL, Chiu SL, Zhu Q, Huganir RL. GRIP1 regulates synaptic plasticity and learning and memory. Proc Natl Acad Sci USA. 2020;117:25085–91.
Mao L, Takamiya K, Thomas G, Lin D, Huganir R. GRIP1 and 2 regulate activity-dependent AMPA receptor recycling via exocyst complex interactions. Proc Natl Acad Sci USA. 2010;107:19038–43.
Takamiya K, Mao L, Huganir R, Linden D. The glutamate receptor interacting protein family of GluR2-binding proteins is required for long term synaptic depression expression in cerebellar Purkinje cells. J Neurosci. 2008;28:5752–5.
Dong H, O’Brien R, Fung E, Lanahan A, Worley P, Huganir R. GRIP: a synaptic PDZ domain-containing protien that interacts with AMPA receptors. Nature. 1997;386:279–84.
Feng W, Shi Y, Li M, Zhang M. Tandem PDZ repeats in glutamate receptor interacting proteins have a novel mode of PDZ domain-mediated target binding. Nat Struct Biol. 2003;10:972–8.
Gainey M, Tatavarty V, Nahmani M, Lin H, Turrigiano G. Activity-dependent synaptic GRIP1 accumulation drives synaptic scaling up in response to action potential blockade. Proc Natl Acad Sci USA. 2015;112:E3590–9.
Mejias R, Adamczyk A, Anggono V, Niranjan T, Thomas G, Sharma K, et al. Gain-of-function glutamate receptor interacting protein 1 variants alter GluA2 recycling and surface distribution in patients with autism. Proc Natl Acad Sci USA. 2011;108:4920–5.
Han M, Mejias R, Chiu S, Rose R, Adamczyk A, Huganir H, et al. Mice lacking GRIP1/2 show increased social interactions and enhancedphosphorylation at GluA2-S880. Behav Brain Res. 2017;321:176–84.
Mejias R, Chiu S, Han M, Rose R, Gil-Infante A, Zhao Y, et al. Purkinje cell-specific Grip1/2 knockout mice show increased repetitive self-grooming and enhanced mGluR5 signaling in cerebellum. Neurobiol Dis. 2019;132:104602.
Niranjan T, Mejias-Estevez R, Han M, Huganir R, Wang T, editors. GRIP2-mediated AMPA signaling defects contribute to autism social behavioral deficits. 64th Annual Meeting of the American Society of Human Genetics; 2014; San Diego, California; October 18-25.
Wu C, Tatavarty V, Jean Beltran P, Guerrero A, Keshishian H, Krug K, et al. A bidirectional switch in the Shank3 phosphorylation state biases synapses toward up- or downscaling. eLife. 2022;11:e74277.
Yong A, Tan H, Zhu Q, Bygrave A, Johnson R, Huganir R. Tyrosine phosphorylation of the AMPA receptor subunit GluA2 gates homeostatic synaptic plasticity. Proc Natl acad Sci USA. 2020;117:4948–58.
Stoner R, Chow M, Boyle M, Sunkin S, Mouton P, Roy S, et al. Patches of disorganization in the neocortex of children with autism. N Engl J Med. 2014;370:1209–19.
Sacai H, Sakoori K, Konno K, Nagahama K, Suzuki H, Watanabe T, et al. Autism spectrum disorder-like behavior caused by reduced excitatory synaptic transmission in pyramidal neurons of mouse prefrontal cortex. Nat Commun. 2020;11:5140.
Fortier A, Meisner O, Nair A, Chang S. Prefrontal circuits guiding social preference: Implications in autism spectrum disorder. Neurosci Biobehav Rev. 2022;141:104803.
Anggono V, Clem R, Huganir R. PICK1 loss of function occludes homeostatic synaptic scaling. J Neurosci. 2011;31:2188–96.
Chung H, Xia J, Scannevin R, Zhang X, Huganir R. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–67.
Xia J, Chung H, Wihler C, Huganir R, Linden D. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/2 and PDZ domain-containing proteins. Neuron. 2000;28:499–510.
Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the Mouse Embryo: A Laboratory Manual. Third ed. Cold Spring Harbor Press; 2003.
Adamczyk A, Mejias R, Takamiya K, Yocum J, Krasnova N, Calderon J, et al. GluA3-deficiency in mice is associated with increased social and aggressive behavior and elevated dopamine in striatum. behav Brain Res. 2012;229:265–75.
Kim Y, Shin J, Kim S. Minhee Analysis Package: an integrated software package for detection and management of spontaneous synaptic events. Mol Brain. 2021;14:138.
Antoine MW, Langberg T, Schnepel P, Feldman DE. Increased excitation-inhibition ratio stabilizes synapse and circuit excitability in four autism mouse models. Neuron. 2019;101:648–61.e4.
Nelson SB, Valakh V. Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron. 2015;87:684–98.
Rein B, Tan T, Yang F, Wang W, Williams J, Zhang F, et al. Reversal of synaptic and behavioral deficits in a 16p11.2 duplication mouse model via restoration of the GABA synapse regulator Npas4. Mol Psychiatry. 2021;26:1967–79.
Yoo T, Cho H, Park H, Lee J, Kim E. Shank3 Exons 14-16 deletion in glutamatergic neurons leads to social and repetitive behavioral deficits associated with increased cortical layer 2/3 neuronal excitability. Front Cell Neurosci. 2019;13:458.
Lee E, Lee J, Kim E. Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol Psychiatry. 2017;81:838–47.
Yi F, Danko T, Botelho SC, Patzke C, Pak C, Wernig M, et al. Autism-associated SHANK3 haploinsufficiency causes Ih channelopathy in human neurons. Science. 2016;352:aaf2669.
Zhang Y, Bonnan A, Bony G, Ferezou I, Pietropaolo S, Ginger M, et al. Dendritic channelopathies contribute to neocortical and sensory hyperexcitability in Fmr1(-/y) mice. Nat Neurosci. 2014;17:1701–9.
Mullins C, Fishell G, Tsien R. Unifying views of autism spectrum disorders: a consideration of autoregulatory feedback loops. Neuron. 2016;89:1131–56.
Steinberg J, Takamiya K, Shen Y, Xia J, Rubio M, Yu S, et al. Targetied in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49:845–60.
Wyszynski M, Kim E, Dunah A, Passafaro M, Valtschanoff J, Serra-Pages C, et al. Interaction between GRIP and Liprin-alpha/SYD2 is required for AMPA receptor targeting. Neuron. 2002;34:39–52.
Dolen G, Bear MF. Role for metabotropic glutamate receptor 5 (mGluR5) in the pathogenesis of fragile X syndrome. J Physiol. 2008;586:1503–8.
Winden K, Ebrahimi-Fakhari D, Sahin M. Abnormal mTOR Activation in Autism. Annu Rev Neurosci. 2018;41:1–23.
Gainey MA, Feldman DE. Multiple shared mechanisms for homeostatic plasticity in rodent somatosensory and visual cortex. Philos Trans R Soc Lond B Biol Sci. 2017;372:20160157.
DeNardo LA, Berns DS, DeLoach K, Luo L. Connectivity of mouse somatosensory and prefrontal cortex examined with trans-synaptic tracing. Nat Neurosci. 2015;18:1687–97.
Anastasiades PG, Carter AG. Circuit organization of the rodent medial prefrontal cortex. Trends Neurosci. 2021;44:550–63.
Van De Werd HJ, Rajkowska G, Evers P, Uylings HB. Cytoarchitectonic and chemoarchitectonic characterization of the prefrontal cortical areas in the mouse. Brain Struct Funct. 2010;214:339–53.
Radnikow G, Feldmeyer D. Layer- and cell type-specific modulation of excitatory neuronal activity in the neocortex. Front Neuroanat. 2018;12:1.
Dong H, Zhang P, Song I, Petralia RS, Liao D, Huganir RL. Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. J Neurosci. 1999;19:6930–41.
Han M, Mejias R, Chiu SL, Rose R, Adamczyk A, Huganir R, et al. Mice lacking GRIP1/2 show increased social interactions and enhanced phosphorylation at GluA2-S880. Behav Brain Res. 2017;321:176–84.
Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–7.
Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Curr Opin Neurobiol. 2000;10:358–64.
Kittler JT, Arancibia-Carcamo IL, Moss SJ. Association of GRIP1 with a GABA(A) receptor associated protein suggests a role for GRIP1 at inhibitory synapses. Biochem Pharmacol. 2004;68:1649–54.
Li RW, Serwanski DR, Miralles CP, Li X, Charych E, Riquelme R, et al. GRIP1 in GABAergic synapses. J Comp Neurol. 2005;488:11–27.
Xia J, Zhang X, Staudinger J, Huganir R. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22:179–87.
Xu J, Xia J. Structure and function of PICK1. Neurosignals. 2006;15:190–201.
Hanley J. PICK1: a multi-talented modulator of AMPA receptor trafficking. Pharmacol Ther. 2008;118:152–60.
Kim C, Chung H, Lee H, Huganir R. Interaction of the AMPA receptor subunit GluR2/3 with PDZ domains regulates hippocampal long-term depression. Proc Natl Acad Sci USA. 2001;98:11725–30.
Perez J, Khatri L, Chang C, Srivastava S, Osten P, Ziff E. PICK1 targets activated protein kinase C alpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci. 2001;21:5417–28.
Lin D, Huganir R. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27:13903–8.
Acknowledgements
This work was supported in part by Simon Foundation (#206683) and NIMH (RO1 MH112808 to R.H. and T.W.; P50 MH100024 to R.H.; R21 MH128765 to J.K.) and a KBRI basic research program (25-BR-02-02 and 25-BR-07-01) through Korea Brain Research Institute funded by Ministry of Science and ICT to J.K.
Author information
Authors and Affiliations
Contributions
M.H., H.L.T., J.K, T.W., and R.L.H. designed research; M.H., H.L.T., R.M., S.-L.C., and J.K. performed research; M.H., H.L.T., J.K. T.W. analyzed data; M.H., H.L.T. J.K., R.L.H. and T.W. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, M., Tan, H.L., Mejías, R. et al. Dysregulated GluA2-Y876 phosphorylation contributes to loss of synaptic upscaling in GRIP1 mutant mice with reduced sociability and increased repetitive behavior. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03415-0
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03415-0