Abstract
The success of ketamine, a dissociative anesthetic and non-competitive N-methyl-D-aspartate receptor (NMDAR) antagonist, as a rapidly acting antidepressant has ignited efforts to identify other novel depression treatments. In recent years, several clinical trials indicated that nitrous oxide (N2O), an inhalational dissociative anesthetic in clinical use for over 150 years, also has rapid and durable antidepressant effects in patients with major depressive disorder (MDD) and treatment resistant major depression (TRMD). N2O is a non-competitive NMDAR inhibitor but acts on NMDARs by mechanisms distinct from ketamine. Cellular and neuronal circuit studies of N2O-induced psychotropic and antidepressant effects are in their infancy and suggest that N2O shares at least some downstream mechanisms with ketamine, while also having unique effects on neurophysiology and signaling. Human neuroimaging and brain network connectivity studies of N2O have begun to identify acute and persisting effects of the drug on brain circuits likely relevant for antidepressant responses. In this review, we highlight the current state of clinical and preclinical research into the effects of N2O and emphasize major unanswered questions, some of which are currently being explored. We emphasize future directions and potential barriers to clinical use of N2O for treatment of patients with psychiatric illnesses.
Similar content being viewed by others
Introduction
Psychiatry is witnessing a revolution in therapeutics fueled by the success of ketamine and esketamine as rapidly acting antidepressants [1]. While traditional antidepressants are helpful, they typically have slow onset of action and limited overall efficacy with 30% or more of patients with major depressive disorder (MDD) failing to respond [2, 3]. By contrast, ketamine produces rapid antidepressant benefits in treatment resistant major depression (TRMD) within hours after a sub-anesthetic intravenous infusion [1]. Intranasal esketamine (the (S+)-ketamine enantiomer) also has rapid antidepressant actions, is FDA approved for TRMD and has an FDA-indication for use in treating depression with suicidal ideation or behavior [1]. Effects of ketamine can persist for days to weeks and outlive the presence of ketamine and its metabolites in brain. Nevertheless, recent meta-analyses indicate that esketamine has significant limitations [4, 5]. Current efforts aim to optimize dose and frequency of ketamine to extend its therapeutic benefits while minimizing its side effects [4].
Based on the success of ketamine and esketamine, there has been interest in identifying alternative pharmacological treatments with rapid onset and longer-lasting improvement, ideally with fewer side effects. Examples of these alternatives include the FDA-approved neuroactive steroids, brexanolone and zuranolone, for postpartum depression [6] and certain experimental agents such as the serotonergic psychedelics including psilocybin [7] and the anti-muscarinic, scopolamine [8]. Additionally, there is interest in repurposing other anesthetic agents based on their rapid modulation of major excitatory (glutamate) and inhibitory (GABA) neurotransmitter systems and the role of these systems in psychiatric illnesses [9,10,11]. In this review, we focus on the inhalational anesthetic, nitrous oxide (N2O), and discuss clinical studies in mood disorders and other potential indications. We also discuss mechanistic studies and compare N2O to ketamine. We end with a discussion of outstanding questions about N2O and its potential use in psychiatry.
Clinical use of nitrous oxide
Although there were early anecdotal reports about antidepressant effects of N2O [12, 13], the first formal N2O clinical trial was conducted following clinical trial successes with ketamine [14]. Ketamine is a non-competitive inhibitor of N-methyl-D-aspartate glutamate receptors (NMDARs) [14], and this mechanism motivated a major change in understanding antidepressant neuroscience. Preclinical studies in the 1990’s found that N2O is also a non-competitive NMDAR antagonist, although working through receptor mechanisms distinct from ketamine [15, 16]. N2O has a long history of safe use in anesthesia and has hypnotic, analgesic and anxiolytic properties over a range of concentrations [17] (Supplemental Fig. 1). Even at high concentrations, N2O is a weak anesthetic that is typically used in combination with other anesthetics for general anesthesia or as a sole agent in outpatient dentistry where its dose is limited to 70% or less to avoid hypoxia. N2O has relatively mild acute side effects that include nausea and vomiting, dizziness, headaches and euphoria [18, 19]. Some patients experience anxiety and dysphoria during administration. Side effects are usually transient and dissipate rapidly once inhalation is stopped. N2O inhibits vitamin B12 and can cause peripheral neuropathy but concerns about this are mostly relevant to prolonged or chronic use [17]. Emergence of psychosis with N2O is rare and typically associated with misuse of high doses of the drug. Dissociative symptoms have been observed with subanesthetic ketamine in healthy volunteers; these symptoms typically occur during infusion and abate within 30-60 min after drug administration is discontinued [20, 21]. Extensive evidence from use of N2O in obstetrics, pediatrics, dentistry, and emergency medicine supports its safe use in vulnerable populations. Akin to ketamine, N2O has abuse potential, but risks appear lower than ketamine [21, 22]. N2O is not scheduled by the U.S. Drug Enforcement Administration (DEA) while ketamine and esketamine are Schedule III and esketamine is subject to an FDA Risk Evaluation and Mitigation Strategy (REMS). Nonetheless, it appears that recreational and abusive N2O use has increased over the past 20 years [22]. Contraindications to N2O use include severe pre-existing vitamin B12 or folate deficiency and conditions with significant air-filled spaces such as middle ear occlusion, pneumothorax, pneumocephalus and bowel obstruction.
Potential advantages of N2O include ease of administration, lack of systemic effects, lack of metabolites, and rapid recovery following inhalation in outpatient settings. Unlike ketamine, N2O does not have systemic side effects such as elevated blood pressure. It has rapid onset and offset of action, features that reflect its low blood solubility [17]. Most patients recover within minutes after N2O inhalation ceases, which allows patients to transport themselves after treatment recovery, in contrast to a minimum 2-hour recovery and alternative transportation required by REMS for esketamine. Because most of the inhaled N2O is exhaled, it has minimal interactions with other drugs and has no metabolites, so once the drug is cleared after inhalation further direct drug action is not a factor. This latter point has important clinical implications and relevance for understanding the mechanisms underlying psychotropic effects - namely that all direct drug effects leading to persisting changes are initiated during drug administration. This contrasts with ketamine, which has a several-hour half-life and produces active metabolites, such as 2,6-hydroxynorketamine and norketamine, which may exert effects beyond the initial dosing period, based on preclinical studies [23,24,25].
Current state of nitrous oxide in psychiatry
As noted, N2O has a long history in anesthesiology and there is more recent interest in its potential use in neuropsychiatry [13]. The first modern trial of N2O for TRMD was reported a decade ago and seven trials for depression have been published to date, most involving subjects with TRMD of varying degrees of refractoriness. The initial trial [26] was a double-blind, placebo-controlled, crossover study of 20 patients with severe TRMD who had failed an average of 8 adequate dose-duration antidepressant trials including 4 individuals who had failed electroconvulsive therapy and 3 who had failed vagus nerve stimulation. Patients were treated for one hour with 50% N2O or placebo as tolerated. Four subjects showed full response ( > 50% reduction in depressive symptoms) at the primary endpoint twenty-four hours after N2O treatment, and three met clinical criteria for remission; only one individual responded to placebo. By design, the crossover between N2O and placebo occurred one week after treatment. Many who experienced N2O antidepressant benefit showed carryover antidepressant effects at that timepoint, suggesting that a subset of TRMD patients experience sustained antidepressant response beyond one week following a single N2O administration. Five subjects had N2O inhalation terminated before one hour because of side effects including nausea, headache and emotional discomfort, while no patients receiving placebo (oxygen + air) had early termination.
This initial trial was followed by a dose-finding study comparing 25% and 50% inhaled N2O for one hour vs. placebo (oxygen + air), based on the premise that a lower concentration of N2O that still inhibits NMDARs [15, 16] would be effective with potentially minimized side effects. This study was also a double-blind, randomized, crossover design with switches occurring at 4-weeks after N2O or placebo [27]. In 24 subjects with moderate to severe TRMD (4-5 treatment failures on average) both 25% and 50% N2O improved depressive symptoms with no apparent antidepressant efficacy difference between the two doses. At the time of crossover, a subset of subjects who received N2O first remained improved while placebo-treated patients had returned to their depressed baseline state. Additionally, the 25% N2O dose demonstrated superior tolerability, with a four-fold lower risk of side effects.
Several other studies have examined N2O effects in TRMD [28]. In a double blind, placebo-controlled trial, Yan et al. [29] studied 20 TRMD participants receiving 50% N2O for one hour compared to 24 placebo-treated TRMD patients. Participants had experienced 2 or more treatment failures in the current episode. Depressive symptoms improved at 2 and 24 h after N2O but did not differ from placebo at one or two weeks. N2O-treated subjects did show improved executive function one week after N2O [30]. In an open label trial, Desmidt et al. [31] studied 20 women with mild TRMD (one treatment failure) using 50% N2O for one hour and found 25% and 45% clinical response at 24 h and 7 days, respectively. Kim et al. [32] reported improvement in 12 participants with bipolar disorder and treatment resistant depression (vs. 13 controls) in a double-blind, placebo-controlled trial using a final dose of 25% N2O for 20 min. These subjects showed improved depression on the day of treatment but not at 24 h.
Ladha et al. recently described a randomized, double blind, parallel design, pilot trial comparing 50% N2O-50% O2 plus intravenous saline infusion (N = 20) to active placebo with intravenous midazolam and 50% O2 (N = 20) in participants with TRMD and two treatment failures in the current episode [33]. N2O and placebo were administered for one hour once weekly for four weeks. At day 42 follow up (3 weeks after the last N2O or placebo treatment), 50% of N2O-treated individuals showed a defined “minimal clinically important” change in depression score (6-point difference) compared to 22.2% of individuals receiving placebo. No participants in the placebo group achieved a clinical response defined as >50% change in depression score while 16.7% of those receiving N2O showed a response (5.6% achieved clinical remission). Adverse effects included nausea and vomiting, headache and anxiety/chest tightness and were described as mild to moderate and transient but more frequent in subjects receiving N2O. One participant receiving N2O required hospitalization for worsening mood symptoms.
Two clinical trials to date have examined N2O for MDD in non-TRMD subjects. Guimaraes et al. [34] conducted a double-blind, parallel design trial of 12 N2O and 11 placebo-treated individuals using 50% N2O administered for one hour twice weekly for four weeks. Subjects receiving N2O showed 92% response and 75% remission after four weeks of treatment. Myles et al. [30] studied 81 patients with MDD treated with either 25% or 50% N2O for 1 h versus 41 with placebo (air/oxygen) administered once weekly for four weeks in a randomized, double-blind trial. MDD subjects in this trial included individuals with TRMD with a median of 6 treatment failures. Although recruitment was limited by the pandemic and the study failed to meet its primary endpoint at 4 weeks, the study met its secondary endpoint and N2O resulted in 38% of participants exhibiting remission by 1 week compared to 13% of controls. Side effects included light-headedness and nausea and were greater in the group receiving 50% N2O.
In summary, several small placebo-controlled studies have reported benefits of N2O in patients with TRMD and MDD (summarized in Table 1). In most studies, benefits were manifest prior to or by 24 h after inhalation and persisted for up to several weeks in some participants. Limitations and challenges in interpreting these studies and potential strategies for improving study designs are discussed below.
Mechanistic studies of nitrous oxide
Effects on human brain networks
There is limited information about the effects of N2O on human brain networks in either healthy or depressed individuals, especially effects persisting beyond the time of drug administration. In healthy subjects, Dai and colleagues [35, 36] examined acute “psychedelic-like” effects (altered consciousness) of 35% N2O for 40 min. Using functional magnetic resonance imaging (fMRI), they observed lower functional connectivity within several brain networks but increased functional connectivity between networks. Notable changes were observed between the temporo-parietal junction and bilateral intraparietal sulci and between the precuneus in the default mode network (DMN) and the left intraparietal sulcus. Other changes included decreased functional differentiation in the frontoparietal and somatomotor networks, with overall flattening of cortical geometry and disruption of network temporal dynamics. Several identified areas, including the DMN, are important for affective processing and have previously been linked to depression [37]. Interestingly, the acute human neuroimaging effects of N2O on within and between network functional connectivity in the Dai et al. studies [35, 36] were similar to what this group also observed with ketamine and LSD despite the drugs having distinctly different molecular actions.
In 16 healthy volunteers, Palanca et al. [38] observed sustained functional connectivity changes induced by one hour exposure to 50% N2O compared to air-oxygen administration. Linearly increasing changes in global functional connectivity within occipital cortex and between visual cortex and the dorsal attention network were observed at 2 and 24 h after N2O exposure. Weaker changes were observed between visual cortex and the frontoparietal (attention) network and DMN. These results demonstrate that the effects of N2O on human brain networks can persist at least 24 h following a single one-hour inhalation but leave unanswered whether these changes relate to effects in depressed patients.
One fMRI study has examined functional connectivity changes in depressed participants treated with N2O. Desmidt et al. [31] found decreased functional connectivity 2-hours after a 1 h 50% N2O exposure between the subgenual anterior cingulate cortex (sgACC) and precuneus in women with mild TRMD who had a clinical response to N2O. In contrast, they observed diminished connectivity between the supracallosal ACC and mid-cingulate cortex in healthy controls and in depressed subjects who failed to respond to N2O exposure. This study also included ultrasound measurements consistent with increased regional cerebral blood flow (CBF). Using arterial spin labelling MRI, Kim et al. [32] observed that lower pre-N2O cerebral blood flow predicted greater N2O response at 24 h in a study of patients with bipolar disorder and refractory depression treated with up to 25% N2O.
A recent human imaging study published in preprint form [39] used a randomized cross-over design with 50% N2O vs. placebo exposure for one hour with treatments spaced one month apart. This study examined functional connectivity changes in five limbic seeds in 14 TRMD and 16 non-depressed participants at baseline and 2- and 24-hours after N2O exposure. The seeds probed networks that participate in MDD/TRMD (DMN, salience, reward, and cingulo-opercular networks). Over 24 h, N2O progressively decreased connectivity in all depression-related networks in subjects with TRMD while connectivity was increased in those same networks in non-depressed controls. Functional connectivity changes were also observed in the dorsal paracingulate cortex, a region known as a “dorsal nexus” that shows hyperconnectivity in MDD [40] (Fig. 1). These N2O-induced functional connectivity changes were not mimicked by placebo. This study supports the idea that N2O persistently reduces functional connectivity beyond 2 h in limbic regions in TRMD and that the effects are related to the presence of depressive illness. The functional connectivity changes moved in opposite directions for the two groups, with N2O-associated decreases in functional connectivity in TRMD and increases in non-depressed controls. These state-dependent (MDD-associated) functional connectivity changes are akin to those observed with ketamine [41].
The connectome ring display shows higher functional connectivity among cingulate cortex seeds (subgenual (Brodman Area (BA) 25), anterior cingulate (BA33), ventral anterior cingulate (BA24), dorsal anterior cingulate (BA32), dorsal posterior cingulate (BA31)) and the salience and default mode networks. Broader connections between seed paths show greater between-group differences. Path maximum t-value = 4.08.
Two studies have examined effects of N2O exposure on electroencephalographic (EEG) measures of brain activity and connectivity in depressed subjects. In patients with TRMD, Shao et al. [42] reported increases in brain connectivity that correlated with changes in depressive symptoms 24 h after N2O. They also observed changes in task-based event related potentials (ERPs) using a face recognition task. In three subjects, Kronenberg et al. [43] observed increased alpha power along with right frontal alpha asymmetry and an overall decrease in EEG vigilance in depressed individuals who responded to N2O treatment.
While data are limited, these fMRI and EEG studies provide tentative support for the idea that N2O has detectable effects on human brain functional connectivity in networks contributing to cognition and emotion. Specific networks studied include the DMN (self-referential thinking), salience and reward networks (emotional valance and motivation) and the cingulo-opercular cognitive control network (error monitoring and alertness) [44, 45]. To date, changes in these networks have not been linked to specific depressive symptom domains following N2O; however, studies are currently underway. Some MDD-specific effects of N2O exposure (reducing connectivity in TRMD while increasing connectivity in controls), have been observed in ketamine neuroimaging studies [41]. There is less information about persisting effects of N2O exposure, but recent studies in TRMD and healthy controls show connectivity changes that last at least 24 h after drug [38, 39]. As noted earlier, clinical TRMD studies have demonstrated sustained mood effects beyond 24 h [26, 27]; whether functional connectivity changes persist beyond 24 h remains to be clarified.
Receptor, ion channel & neural circuitry effects in preclinical studies
N2O is a non-competitive NMDAR antagonist [15], acting by mechanisms distinct from ketamine. While ketamine inhibits open NMDAR channels through sites within the ion channel pore and results in a use- and voltage-dependent form of block, effects of N2O are neither strongly voltage- nor use-dependent. Consistent with this, ketamine speeds decay of NMDAR-gated currents including excitatory postsynaptic currents, a feature not shared by N2O [16]. It is presently unknown where or how N2O acts within the NMDAR complex, and unlike ketamine there is little information about effects on NMDARs of varied subunit composition. However, certain behavioral changes induced by N2O have been linked to effects on NMDARs [46].
N2O also weakly inhibits the AMPA class of glutamate receptors and weakly potentiates inhibitory GABAARs [16]. The latter effect may contribute to anxiolysis and may be mediated by the benzodiazepine binding site on GABAARs [47]. The drug also has weak effects on GABA receptors comprised of rho subunits (sometimes called GABACRs) [48]. N2O is a partial inhibitor of low voltage activated, Cav3.2 but not Cav3.1, T-type Ca2+ channels [49]. T-channel effects do not appear to be shared by ketamine. Because T-currents and NMDARs contribute to behaviorally important burst firing in lateral habenula, inhibition of T-currents by N2O and/or NMDAR antagonism (both N2O and ketamine) could suppress this burst firing and contribute to antidepressant-like changes [50, 51]. The lateral habenula, a key relay linking limbic regions with midbrain monoaminergic systems, encodes negative valence signals. Aberrant activity in this region participates in depressive-like symptoms in preclinical studies, including aversive and anhedonia-like behaviors [52,53,54]. Human studies provide mixed support for findings in habenula in mood disorders but indicate modulation by ketamine [55, 56].
N2O activates α-adrenergic receptors [57, 58] and weakly inhibits serotonin-3 (5HT3) and certain nicotinic acetylcholine receptors (α4β2 and α4β4) [48]. The drug also activates 2-pore domain TREK-1 potassium channels [59]. An intriguing effect that could contribute to psychiatric and analgesic properties is N2O’s ability to stimulate certain opioid receptors [60]. Opioid receptor effects of N2O preferentially involve κ-opioid receptors with lesser contribution from δ-receptors [61, 62] and are observed at concentrations used to treat depression, and that affect NMDARs [63,64,65,66]. In rats, N2O produces acute analgesic effects via opioid receptors. However, a single administration of 50% N2O for 75 min can dampen neuropathic pain in a chronic sciatic nerve injury model for several weeks by mechanisms independent of acute opioid receptor effects and possibly involving NMDAR inhibition [67]. Effects of ketamine on the opioid system have also been described, and μ-type opioid receptors may contribute to antidepressant activity in humans [4] and changes in glutamate signaling based on magnetic resonance spectroscopy measures [68]. Preclinical studies with ketamine suggest that the opioid system is more likely permissive and not sufficient for antidepressant-like changes [69]. The role of the endogenous opioid system in the effects of ketamine in concert with NMDARs remains an important area of active investigation [70].
Mechanistic preclinical studies of N2O on rodent brain circuits are limited and have been informed by work on ketamine. Despite its NMDAR antagonism and the role that NMDAR activation plays in triggering synaptic plasticity, ketamine persistently enhances glutamatergic transmission in several brain regions [71,72,73]. This synaptic enhancement occurs rapidly and involves increased expression of synaptic AMPARs and structural changes in dendrites [71]. Potentiating effects of ketamine involve several intracellular signaling systems including mechanistic target of rapamycin (mTOR) [71], brain-derived neurotrophic factor (BDNF) and its receptor tropomyosin receptor kinase B (TrkB) [72,73,74,75], and nitric oxide synthase (NOS) [76,77,78]. While effects on nitric oxide (NO) synthesis have been observed in preclinical studies, it is not clear how these effects translate to antidepressant actions in humans based on a study using sodium nitroprusside as a NO donor [79].
Less is known about effects of N2O on circuit function, but several findings are consistent with ketamine’s effects. In adult rat hippocampal slices, acute perfusion of 30% N2O causes initial depression of AMPAR-mediated synaptic responses during drug administration (unlike ketamine) [78]. This initial depression may reflect changes in tissue oxygenation, weak inhibition of AMPARs [16] and/or presynaptic effects [80, 81]. Following N2O perfusion, there is a rapid and persistent enhancement of glutamate transmission that affects both AMPAR and NMDAR synaptic responses. N2O engages similar signaling systems as ketamine. Early events include activation of NMDARs that remain unblocked during acute drug administration. Thus, complete NMDAR inhibition with a high concentration of a standard competitive NMDAR antagonist during N2O administration prevents synaptic enhancement [71, 78, 82]. Other shared mechanisms include mTOR, NOS and TrkB receptors [78, 83]. A key question concerns how ketamine and N2O activate unblocked NMDARs. For ketamine, this appears to involve local disinhibition in which ketamine preferentially inhibits NMDARs on interneurons, possibly NMDARs expressing GluN2B or GluN2C/D subunits [84,85,86,87]. Akin to ketamine, N2O disinhibits the CA1 hippocampal circuit contributing to changes in local excitation [88]. A potential mechanism contributing to disinhibition is the ability of N2O to weakly enhance synaptic GABAA receptor inhibition of hippocampal interneurons [16]. A challenge in relating these effects of N2O on CA1 transmission to antidepressant actions is that these studies were done in naïve (unstressed) rodents. Thus, the observed enhanced transmission may mirror the increased functional connectivity observed in healthy human controls after N2O exposure [38, 39], in contrast to the diminished connectivity in mood-related brain circuits observed in depressed subjects [31, 39].
In other preclinical studies, N2O increases expression of the bdnf gene and immediate early genes cFos and Arc while promoting phosphorylation of TrkB receptors [89]. Transcriptional changes occur acutely and are followed by phosphorylation of TrkB, glycogen synthase kinase 3 and mitogen activated protein kinase (MAPK). These phosphorylation events occur after N2O exposure at a time when cortical EEGs show rebound homeostatic slow waves. A recent study in mice linked post-N2O-induced changes in TrkB activation, EEG oscillations, glucose utilization and antidepressant-like effects to decreases in body temperature [90]. N2O exposure also rapidly increases calcium signals in mPFC that precede changes in cortical EEG activity detected in both sleep (increased slow wave activity) and awake states (increased gamma oscillations) ([91], preprint). In healthy human participants (N = 7 men), 50% N2O for 20 min increased EEG gamma power during administration with decreases in alpha and beta band power. In the 10 min following N2O, increases in gamma oscillations persisted while increases in theta but not delta frequency power were also observed, differing from observations in mice [92]. Other evidence indicates that N2O activates NOS early, promoting BDNF expression, antidepressant-like activity [93] and possibly anxiolytic-like effects [94]. Changes in nitric oxide could also contribute to the effects on body temperature noted above [90]. The time course of these latter effects may differ from those of ketamine because ketamine stimulates early translation of the bdnf gene followed by subsequent transcriptional changes [87].
Hippocampal synaptic enhancement by N2O occludes potentiation by ketamine, supporting overlapping mechanisms. However, LTP induced by electrical stimulation of hippocampal synapses remains intact after N2O administration [78]. AMPARs play a key role in synaptic potentiation by ketamine, acting both as a trigger for enhancement and as a mediator of potentiation via increased receptor expression [95]. In contrast, induction of synaptic enhancement by N2O does not require AMPAR activation [78]. This latter finding likely reflects differences in how the drugs inhibit NMDARs. With ketamine, NMDAR channels must open for block to occur. Hence, complete block of AMPARs dampens the effects of ketamine on NMDAR channels by preventing AMPAR-mediated depolarization. N2O does not require channel opening to inhibit NMDARs [16]. Another important difference is that N2O, unlike ketamine, is cleared rapidly once administration ends and has no active metabolites.
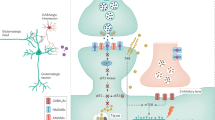
A recent preclinical study in medial prefrontal cortex (mPFC) further elucidates N2O’s cellular and circuit mechanisms, highlighting a potentially novel effect that may differ from ketamine [96]. In this study, 50% N2O for one hour produced rapid activation of layer 5 (L5) pyramidal neurons in wild-type, chronically stressed mice. This L5 activation reversed stress-induced hypoactivity in L5 and was associated with antidepressant-like behavioral effects. Enhanced L5 activity involved disinhibition via effects on somatostatin- and parvalbumin-expressing interneurons resulting from activation of interneurons expressing vasoactive intestinal peptide (VIP) (Fig. 2). Importantly, L5 activation involved inhibition of SK2 potassium channels rather than NMDARs; both L5 pyramidal neurons and VIP interneurons express SK2 channels. SK2 channels contribute to the medium afterhyperpolarization (AHP) that follows action potential firing and help to regulate neuronal excitability [97,98,99]. While NMDARs did not directly trigger L5 changes, NMDARs contributed to persistent activity changes following N2O exposure. Importantly, this study raises questions about the primary role of NMDARs in antidepressant-like effects triggered in mPFC. Similarly, there are remaining questions about the role of NMDARs in the effects of ketamine considering metabolites that have antidepressant-like properties but marginal NMDAR effects based on preclinical studies [1, 25, 100].
In hippocampus, N2O disinhibits the CA1 circuit likely by inhibiting NMDARs on GABAergic interneurons, resulting in increased activity of pyramidal (glutamatergic) neurons (Fig. 2). This triggers activation of unblocked postsynaptic NMDARs and stimulates downstream signaling involving BDNF, NOS and mTOR, along with MAPK, ERK and Akt. In mPFC, inhibition of SK2 channels on VIP interneurons triggers disinhibition via somatostatin (SST) and parvalbumin (PV)-expressing interneurons, while inhibition of SK2 on L5 pyramidal neurons contributes to increased excitability. The net result is persistently enhanced excitatory function. This figure was made with BioRender.
Also in PFC, N2O and ketamine promote transcription of multiple genes, including dual function phosphatases that regulate MAPKs [101]. In this study, N2O produced more widespread effects on gene expression, while ketamine, but not N2O, increased spiking of pyramidal neurons and increased gamma frequency oscillations. In other rodent studies, 50% N2O transiently increases EEG signal complexity and high gamma band power associated with increased acetylcholine (ACh) levels in prefrontal and parietal regions. These early changes last about 12 min and are followed by reduced complexity, weaker frontoparietal high gamma band power and lower ACh levels over a 1-hour drug exposure [102]. Effects of N2O differed from subanesthetic ketamine, which showed sustained increases in these parameters over the period of drug exposure. Again, these findings suggest potentially important differences between N2O and ketamine that remain to be clarified and fully understood (Table 2).
In a preclinical study in mice using a drug administration paradigm designed to mimic repeated abusive drug use (20% N2O daily for 4 consecutive days), N2O was found to upregulate transmission in the ventral tegmental area-nucleus accumbens (VTA-NAc) reward pathway by effects on BDNF and D1 dopamine receptor activity [103]. Whether similar changes occur in this reward/motivation circuit with N2O dosing used for depression is unknown. In recent studies, ketamine has been shown to exert anti-anhedonia-like effects via VTA-NAc signaling [73].
Key questions about nitrous oxide
Clinical questions
The studies outlined in this review highlight ongoing progress in understanding N2O as a potential psychiatric treatment. It is clear, however, that there are presently more questions than answers. Here we will enumerate some of the major issues and potential solutions.
-
1.
Clinical evidence supporting antidepressant effects of N2O is based on a small number of trials with relatively few participants. Thus, there is major need for larger, multi-site, double-blind, placebo-controlled trials potentially allowing analysis of clinical subgroups of patients, including non-resistant MDD, bipolar depression (including bipolar patients with treatment resistant depression) and unipolar TRMD. To date, there is little information about effects of N2O in these latter individuals. Further work on N2O dosing is needed to determine whether fixed, titrated, or multidose schedules are most effective and if titration helps to minimize side effects [104]. The duration and magnitude of N2O exposure can be much more easily controlled compared to ketamine, perhaps permitting more individualized treatment protocols for specific patients.
-
2.
Antidepressant benefits of N2O exposure can be sustained for weeks or longer. This durability varies among participants and complicates studies using crossover designs comparing N2O and placebo exposure as there are persisting effects if crossover is scheduled too early. In trials to date some patients show persisting effects of N2O a month or longer following a single administration. To avoid crossover complications parallel study designs are critical for future studies.
-
3.
More information is needed about the duration of mood effects. As noted, some improvements in depression can last a month or more. However, this phenomenon has not been studied systematically. Effects of N2O likely fade over time, as they do with ketamine. Thus, another challenge is to identify strategies to prolong the therapeutic benefits, by optimizing dose size and frequency. With ketamine, it appears that repeated administrations, adjunctive rapamycin or the addition of concomitant psychotherapy may help [4]. A major trend in studies of psychedelic treatments is the concomitant use of psychotherapies with psychedelics. Studies suggest that combined treatments may provide better outcomes and support to patients receiving agents that cause dissociation [105, 106]. Most psychedelic studies to date employ psychotherapy, so whether this is essential is still debated [107]. However, this combined treatment model could be an opportunity in N2O therapy. A recent preclinical study indicates that inhibition of dual-specificity phosphatase 6 (DUSP6) increases the activity of extracellular signal-related kinase (ERK) downstream to BDNF and enhances synaptic effects of ketamine in hippocampus while prolonging antidepressant-like effects for up to 2 months [108]. Similar effects in humans would be clinically important and likely have relevance for N2O given prior work showing upregulation of DUSPs by N2O in rodents [101].
-
4.
Multiple clinical trials have used placebo control, typically with other gases (air + oxygen). It is unclear whether this placebo is satisfactory and how well blinding is maintained. Recent ketamine trials have used intravenous midazolam as a control. Midazolam or a psychoactive variant could be considered in N2O trials as an active control for sedating effects of the drug. A recent pilot trial used midazolam as a placebo and showed beneficial effects of N2O [33]. Alternatively, low doses of N2O (10% or less) could be explored to determine a minimum effective antidepressant dose. Exploring additional N2O doses could also help characterize side effects in psychiatric settings. We note that in studies to date the use of air + oxygen placebo has been associated with significant placebo responses that appear to dissipate by two weeks after inhalation [27].
-
5.
Other extant questions concern whether N2O shares ketamine’s apparent beneficial effects on suicidality and the possible use of N2O in other psychiatric disorders. To date, only one secondary analysis [109] has described effects of N2O on suicidal ideation and provides tentative support for possible anti-suicidal effects. Systematic studies examining N2O for psychiatric indications other than depression are lacking. A small open label study [110] found that two of three patients with severe post-traumatic stress disorder (PTSD) responded to 50% N2O. This observation is consistent with an experimental study reporting that N2O exposure can speed reduction of intrusive memories [111]. N2O administered at 50% for 30 min may also interfere with memory reconsolidation [112] as has been observed in a study of alcohol-related memories in subjects with hazardous drinking [113]. These areas require further exploration in larger, well-designed studies. N2O should also be studied in primary anxiety disorders, given its reported anxiolytic properties [92, 114,115,116]. There are presently multiple trials listed in ClinicalTrials.gov examining N2O for treatment of MDD, bipolar depression, PTSD, obsessive compulsive disorder and acute suicidal ideation. Results from these trials should inform future studies.
Mechanistic questions
There are numerous unanswered questions about how N2O works as an anesthetic, analgesic and pharmacotherapeutic across different concentrations and exposure durations and frequency. Here we enumerate several opportunities.
-
1.
Mechanisms underlying N2O effects on NMDARs remain uncertain and it is unknown whether N2O has preferential actions on NMDAR subtypes as reported for ketamine [24]. N2O-mediated NMDAR antagonism differs from ketamine and N2O is not an open channel blocker. Thus, direct mechanistic comparisons to ketamine are limited. Based on studies examining N2O block of T-type calcium channels, it is possible that N2O acts on NMDARs via a form of redox modulation [117,118,119].
-
2.
N2O, like ketamine, persistently enhances excitatory transmission in hippocampus and mPFC, and local circuit disinhibition contributes to these effects. Although partial inhibition of NMDARs triggers synaptic enhancement in hippocampus [78], this effect is less clear in cortex, where SK2 channels play a role [96]. Understanding other regional mechanistic differences will also be important [120]. For ketamine, the role of NMDAR inhibition as an upstream trigger of antidepressant-like effects has been questioned based on the effects of NMDAR-inactive metabolites [25]. Other work has linked persistent blocking effects of ketamine on NMDAR channels to antidepressant-like effects [100]. N2O does not appear to have persisting ion channel effects and drug metabolites are unlikely to be involved in its actions. Likewise, mechanistic triggers for N2O other than NMDARs have been proposed [60, 96]. Although some data favor a common upstream NMDAR trigger [78], mechanisms of ketamine’s NMDAR-inactive metabolites may converge with those of N2O downstream.
-
3.
Further work on downstream messengers and changes in neuronal structure and gene expression is needed to understand the drug’s persistent effects. N2O increases cell proliferation (neurogenesis) in the dentate gyrus. This effect could contribute to slower developing and longer-lived structural plasticity [121]. It is presently unclear how or whether enhancement of excitatory transmission contributes to N2O’s behavioral and therapeutic effects, although there is tentative support for enhanced synaptic efficacy contributing to ketamine’s effects [24, 122,123,124,125]. It is also important to understand whether mechanisms underlying dissociative effects of N2O and ketamine contribute to their therapeutic results [126].
-
4.
There are substantial challenges in studying N2O in preclinical settings. N2O can be difficult to administer, particularly in ex vivo tissue slices where changes in oxygenation can confound results as N2O concentrations are increased. On the other hand, N2O can be administered in vivo at relevant subanesthetic doses, which permits studies of network functional changes.
-
5.
There is limited information about persisting effects of N2O on human brain networks. Preliminary work suggests that N2O-associated network changes can be subtle and may require large sample sizes to accurately identify. These efforts would benefit from use of precision functional mapping (PFM) methods to better define changes in individual subjects relevant to therapeutic and adverse effects [127, 128]. It is intriguing that imaging studies to date suggest that N2O may have differential persisting circuit effects in healthy controls (increased connectivity) compared to TRMD subjects (diminished connectivity) [31, 38, 39]. Studies of brain oscillations including measures of criticality and stabilization could provide additional insights into mechanisms, given well-characterized effects of ketamine on brain rhythms [129,130,131] and preclinical studies indicating that N2O increases slow wave activity following inhalation [89,90,91,92]. N2O may also acutely increase beta- and gamma-band EEG power [132].
Summary & conclusions
Although these are early days for psychiatric use of N2O, accumulating data tentatively support N2O as having efficacy for depressive disorders. To date, most studies involve TRMD, and this population may prove to be a niche for the drug, akin to ketamine. Whether N2O is as effective as ketamine in TRMD remains to be determined. Mechanistic studies of N2O and ketamine are progressing and indicate both shared mechanisms and some unique effects. It is increasingly clear that traditional anesthetics are altering the therapeutic landscape in psychiatry [133]. Ketamine is the leading example, but N2O merits further consideration. Also, certain GABAergic neurosteroids such as allopregnanolone (brexanolone) are endogenous modulators that have served as templates for some anesthetics [134] and have efficacy in neuropsychiatry [9, 135, 136]. Other GABAergic anesthetics, including propofol and isoflurane, are being considered [10, 11, 133].
If larger clinical trials support therapeutic benefits, it will be instructive to see how N2O fits into psychiatry. N2O is relatively easy to administer and has a long history of safe sub-anesthetic clinical use, especially in outpatient dentistry. It is conceivable that N2O could be administered in sub-anesthetic doses in psychiatric outpatient practices, although there will likely be regulatory requirements for its use as in dentistry where practitioners must take a certification course [17]. In hospital settings, N2O often falls under the purview of anesthesiology and sedation committees. These features may limit availability for use by psychiatrists but are not insurmountable given the practical ease of drug administration. Lack of FDA approval for the repurposed drug could also be a deterrent as it is with ketamine [137]. Improvements in medical devices and methods for gas delivery are opportunities to enhance patient tolerability and acceptance during inhalation.
The advent of rapidly acting antidepressant treatments with novel mechanisms is providing new options for patients. Many important questions remain [4], however, including the sustainability of effects and risks and benefits of longer-term and repeated use (potentially “perpetual use”) of these agents as is being studied with esketamine [138]. How future practice adopts N2O will be determined by its effectiveness, duration of effects, and ease of use, relative to its side effects, safety, risks and cost.
References
Johnston JN, Kadriu B, Kraus C, Henter ID, Zarate CA Jr. Ketamine in neuropsychiatric disorders: an update. Neuropsychopharmacol. 2024;49:23–40.
Conway CR, George MS, Sackeim HA. Toward an evidence-based, operational definition of treatment-resistant depression. When enough is enough. JAMA Psychiatry. 2017;74:9–10.
McIntyre RS, Alsuwaidan M, Baune M, Berk M, Demyttenaere K, Goldberg JF, et al. Treatment-resistant depression, prevalence, detection, management, and investigational interventions. World Psychiatry. 2023;22:394–412.
Schatzberg AF, Mathew SJ. The why, when, how, and so what of so-called rapidly acting antidepressants. Neuropsycopharmacol. 2024;49:189–96.
Fountoulakis KN, Saitis A, Schatzberg AF. Esketamine treatment for depression in adults: a PRISMA systematic review and meta-analysis. Am J Psychiatry. 2025;182:259–75.
Patterson R, Balan I, Morrow AL, Meltzer-Brody S. Novel neurosteroid therapeutics for post-partum depression: perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacol. 2024;49:67–72.
Mitchell JM, Anderson BT. Psychedelic therapies reconsidered: compounds, clinical indications and cautious optimism. Neuropsychopharmacol. 2024;49:96–103.
Drevets WC, Bhattacharya A, Furey ML. The antidepressant efficacy of the muscarinic antagonist scopolamine: past findings and future directions. Adv Pharmacol. 2020;89:357–85.
Kalmoe MC, Janski AM, Zorumski CF, Nagele P, Palanca BJ, Conway CR. Ketamine and nitrous oxide: the evolution of NMDA receptor antagonists as antidepressant agents. J Neurol Sci. 2020;412:116778. https://doi.org/10.1016/j.jns.2020.116778.
Wu G, Xu H. A synopsis of multitarget therapeutic effects of anesthetics on depression. Eur J Pharmacol. 2023;957:176032.
Smith Breault M, Orguc S, Kwon O, Kang GH, Tseng B, Schreier DR, et al. Anesthetics as treatments for depression: clinical insights and underlying mechanisms. Annu Rev Neurosci. 2025;48:103–24.
Milne B. Nitrous oxide (laughing gas) inhalation as an alternative to electroconvulsive therapy. Med Hypotheses. 2010;74:780–1.
Gillman MA. Mini review: A brief history of nitrous oxide (N2O) use in neuropsychiatry. Curr Drug Res Rev. 2019;11:12–20.
Zorumski CF, Nagele P, Mennerick S, Conway CR. Treatment-resistant major depression: rationale for NMDA receptors as targets and nitrous oxide as therapy. Front Psychiatry. 2015;6:172. https://doi.org/10.3389/fpsyt.2015.00172.
Jevtovic-Todorovic V, Todorovic SM, Mennerick S, Powell S, Dikranian K, Benshoff N, et al. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nature Medicine. 1998;4:460–3.
Mennerick S, Jevtovic-Todorovic V, Todorovic SM, Shen W, Olney JW, Zorumski CF. Effect of nitrous oxide on excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 1998;18:9716–26.
Nagele P, Zorumski CF, Conway C. Exploring nitrous oxide as treatment of mood disorders. Basic concepts. J Clin Psychopharmacol. 2018;38:144–8.
Onody P, Gil P, Hennequin M. Safety of inhalation of a 50% nitrous oxide/oxygen premix. A prospective survey of 35,828 administrations. Drug Safety. 2006;29:633–40.
Qui Y, Li L, Duan A, Wang M, Xie M, Chen Z, et al. The efficacy and tolerability of inhaled nitrous oxide in major depressive disorder: a systematic review and meta-analysis. Psychopharmacol (Berl). 2023;240:2033–43.
Beck K, Hindley G, Borgan F, Ginestet C, McCutcheon R, Brugger S, et al. Association of ketamine with psychiatric symptoms and implications for its therapeutic use and for understanding schizophrenia. JAMA Network Open. 2020;3:e204693.
Cousaert C, Heylens G, Audenaert K. Laughing gas abuse is no joke. An overview of the implications for psychiatric practice. Clin Neurol Neurosurg. 2013;115:859–62.
Back S, Kroon E, Colyer-Patel K, Cousijn J. Does nitrous oxide addiction exist? An evaluation of the evidence for the presence and prevalence of substance use disorder symptoms in recreational nitrous oxide users. Addiction. 2024;119:609–18.
Highland JN, Morris PJ, Konrath KM, Riggs LM, Hagen NR, Zanos P, et al. Hydroxynorketamine pharmacokinetics and antidepressant behavioral effects of (2,6)- and (5R)-methyl-(2R,6R)-hydroxynorketamines. ACS Chem Neurosci. 2022;13:510–23.
Krystal JH, Kaye AP, Jefferson S, Girgenti MJ, Wilkinson ST, Sanacora G, et al. Ketamine and the neurobiology of depression: toward next-generation rapid-acting antidepressant treatments. Proc Natl Acad Sci. 2023;49:e2305772120.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6.
Nagele P, Duma A, Kopec M, Gebara MA, Parsoei A, Walker M, et al. Nitrous oxide for treatment-resistant depression: a proof-of-concept trial. Biol Psychiatry. 2015;78:10–182015.
Nagele P, Palanca BJ, Gott B, Brown F, Barnes L, Nguyen T, et al. A phase 2 trial of nitrous oxide for treatment-resistant major depression. Science Transl Med. 2021;13:eabe1376.
Liu H, Kerzner J, Demchenko I, Wijeysundera DN, Kennedy SH, Ladha KS, et al. Nitrous oxide for the treatment of psychiatric disorders: a systematic review of the clinical trial landscape. Acta Psychiatr Scand. 2022;146:126–38.
Yan D, Liu B, Wei X, Ou W, Liao M, Ji S, et al. Efficacy and safety of nitrous oxide for patients with treatment-resistant depression, a randomized controlled trial. Psychiatry Res. 2022;317:114867.
Liu J, Zhao X, Wei X, Yan D, Ou W, Liao M, et al. Empirical evidence for the neurocognitive effect of nitrous oxide as an adjunctive therapy in patients with treatment resistant depression: a randomized controlled study. Psychiatry Res. 2023;326:115326.
Desmidt T, Dujardin P-A, Andersson F, Brizard B, Remenieras J-P, Gissot V, et al. Changes in cerebral connectivity and brain tissue pulsations with the antidepressant response to an equimolar mixture of oxygen and nitrous oxide: an MRI and ultrasound study. Mol Psychiatry. 2023;28:3900–8.
Kim WSH, Dimick MK, Omrin D, Mitchell RHB, Riegert D, Levitt A, et al. Proof-of-concept randomized controlled trial of single-session nitrous oxide for refractory bipolar depression: focus on cerebrovascular target engagement. Bipolar Disord. 2023;25:221–32.
Ladha KS, Lee J, Mattina GF, Pazmino-Canizares J, Wijeysundera DN, Nezhad FG, et al. Sustained mood improvement with laughing gas exposure (SMILE): a randomized, placebo-controlled pilot trial of nitrous oxide for treatment-resistant depression. BJ Psych Open. 2025;11:e208. https://doi.org/10.1192/bjo.2025.10823. 1-7, 2025.
Guimaraes MC, Guimaraes TM, Hallak JE, Abrao J, Machado-de-Sousa JP. Nitrous oxide as an adjunctive therapy in major depressive disorder: a randomized controlled double-blind pilot trial. Braz J Psychiatry. 2021;43:484–93. https://doi.org/10.1590/1516-4445-2020-1543.
Dai R, Huang Z, Larkin TE, Tarnal V, Picton P, Vlisides PE, et al. Psychedelic concentrations of nitrous oxide reduce functional differentiation in frontoparietal and somatomotor cortical networks. Commun Biol. 2023;6:1284. https://doi.org/10.1038/s42003-023-05678-1.
Dai R, Larkin TE, Huang Z, Tarnal V, Picton P, Vlisides PE, et al. Classical and non-classical psychedelic drugs induce common network changes in human cortex. Neuroimage. 2023;273:120097.
Gratton C, Kraus BT, Greene DJ, Gordon EM, Laumann TO, Nelson SM, et al. Defining individual-specific functional neuroanatomy for precision psychiatry. Biol Psychiatry. 2020;88:28–39.
Palanca BJA, Conway CR, Zeffiro T, Gott BM, Nguyen T, Janski A, et al. Persistent brain connectivity changes in healthy volunteers following nitrous oxide inhalation. Biol Psychiatry Global Open. Science. 2023;3:698–704.
Conway CR, Palanca BJA, Zeffiro T, Bott BM, Brown F, de Leon V, et al. Nitrous oxide alters functional connectivity in medial limbic structures in treatment-resistant major depression. MedRxiv. [Preprint]. 2024. https://doi.org/10.1101/2024.08.12.24311729. https://medrxiv.org/cgi/content/short/2024.08.12.24311729v1.
Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci. 2010;107:11020–5.
Alexander L, Hawkins PCT, Evans JW, Mehta MA, Zarate CA Jr. Preliminary evidence that ketamine alters anterior cingulate resting-state functional connectivity in depressed individuals. Transl Psychiatry. 2023;13:371. https://doi.org/10.1038/s41398-023-02674-1.
Shao X, Yan D, Kong W, Sun S, Liao M, Ou W, et al. Brain function changes reveal rapid antidepressant effects of nitrous oxide for treatment-resistant depression: evidence from task-state EEG. Psychiatry Res. 2023;322:115072.
Kronenberg G, Muller A, Seifritz E, Olbrich S. A distinct pattern of EEG and ECG changes associated with inhalational nitrous oxide’s rapid antidepressant effects. Eur Arch Psych Clin Neurosci. 2023;273:1395–7. https://doi.org/10.1007/s00406-022-01502-9.
Scalabrini A, Vai B, Poletti S, Damiani S, Mucci C, Colombo C, et al. All roads lead to the default-mode network – global source of DMN abnormalities in major depressive disorder. Neuropharmacol. 2020;45:2058–69.
Kaiser RH, Andrews-Hanna JR, Wager TD, PIzzagalli DA. Large scale network dysfunction in major depressive disorder: a meta-analysis of resting state functional connectivity. JAMA Psychiatry. 2015;72:603–11.
Nagele P, Metz LB, Crowder CM. Nitrous oxide (N2O) requires the N-methyl-D-aspartate receptor for its action in Caenorhabditis elegans. Proc Natl Acad Sci. 2004;101:8791–6.
Czech DA, Quock RM. Nitrous oxide induces an anxiolytic-like effect in the conditioned defensive burying paradigm, which can be reversed with a benzodiazepine receptor blocker. Psychopharmacol. 1993;113:211–6.
Yamakura Y, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology. 2000;93:1095–101.
Todorovic SM, Jevtovic-Todorovic V, Mennerick S, Perez-Reyes E, Zorumski CF. Ca(v)3.2 channel is a molecular substrate for inhibition of T-type calcium currents in rat sensory neurons by nitrous oxide. Mol Pharmacol. 2001;60:603–10.
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554:317–22.
Cui Y, Hu S, Hu H. Lateral habenula burst firing as a target of the rapid antidepressant effects of ketamine. Trends Neurosci. 2019;42:179–91.
Browne CA, Hammack R, Lucki I. Dysregulation of the lateral habenula in major depressive disorder. Front Synaptic Neurosci. 2018;10:46. https://doi.org/10.3389/fnsyn.2018.00046.
Yang Y, Wang H, Hu J, Hailan H. Lateral habenula in the pathophysiology of depression. Curr Op Neurobiol. 2018;48:90–96.
Gold PW, Kadriu B. A major role for the lateral habenula in depressive illness: physiological and molecular mechanisms. Front Psychiatry. 2019;10:320. https://doi.org/10.3389/fpsyt.2019.00320.
Chen C, Wang W, Yu T, Feng W, Xu Y, Ning Y, et al. Habenular functional connections are associated with depression state and modulated by ketamine. J Affective Dis. 2024;345:177–85.
Fortin J-S, Lafleur M, Parisien C, Hetu S. The habenula in mood disorders: a systematic review of human studies. Mol Psychiatry. 2025;30:4948–70.
Sawamura S, Kingery WS, Davies MF, Agashe GS, Clark JD, Kobilka BK, et al. Antinociceptive action of nitrous oxide is mediated by stimulation of noradrenergic neurons in the brainstem and activation of 2B adrenoceptors. J Neurosci. 2000;20:9242–51.
Orii R, Ohashi Y, Gui T, Nelson LE, Hashimoto T, Maze M, et al. Evidence for the involvement of spinal cord alpha 1 adrenoceptors in nitrous oxide-induced antinociceptive effects in Fischer rats. Anethesiology. 2002;97:1458–65.
Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-pore-domain K+ channels are a novel target for the anesthetic gases xenon, nitrous oxide, and cyclopropane. Mol Pharmacol. 2004;65:443–52.
Gilman MA. Opioid properties of nitrous oxide and ketamine contribute to their antidepressant actions. Int J Neuropsychopharmacol. 2021;24:892–3.
Fukagawa H, Koyama T, Kududa K. κ-Opioid receptor mediates the antinociceptive effect of nitrous oxide in mice. Br J Anaesth. 2014;113:1032–8.
Quock RM, Walczak CK, Henry RJ, Chen DC. Effect of subtype-selective opioid receptor blockers on nitrous oxide antinociception in rats. Pharmacol Res. 1990;22:351–7.
Daras C, Cantrill RC, Gillman MA. 3[H]-Naloxone displacement: evidence for nitrous oxide as an opioid agonist. Eur J Pharmacol. 1983;89:177–8.
Ori C, Ford-Rice F, London ED. Effects of nitrous oxide and halothane on mu and kappa opioid receptors in guinea pig brain. Anesthesiology. 1989;70:541–4.
Fang F, Guo TZ, Davies MF, Maze M. Opiate receptor in the periaqueductal gray mediate analgesic effects of nitrous oxide in rats. Eur J Pharmacol. 1997;336:137–41.
Ohashi Y, Guo TY, Orii R, Maze M, Fujinaga M. Brain stem opioidergic and GABAergic neurons mediate the antinociceptive effect of nitrous oxide in Fischer rats. Anesthesiology. 2003;99:947–54.
Ben Boujema M, Labouryras E, Pype J, Bessiere B, Simonnet G. Nitrous oxide persistently alleviates pain hypersensitivity in neuropathic rats: a dose-dependent effect. Pain Res Manag. 2015;20:309–15.
Jelen LA, Lythgoe DJ, Stone JM, Young AH, Mehta MA. Effect of naltrexone pretreatment on ketamine-induced glutamatergic activity and symptoms of depression: a randomized crossover study. Nature Medicine. 2025;31:2958–66.
Klein ME, Chandra J, Sheriff S, Malinow R. Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc Natl Acad Sci. 2020;117:2656–62.
Levinstein MR, Budinich RC, Bonaventura J, Schatzberg AF, Zarate CA Jr, Michaelides M. Redefining ketamine pharmacology for antidepressant action: synergistic NMDA and opioid receptor interactions? Am J Psychiatry. 2025;182:247–58.
Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.
Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-F, et al. NMDA receptor blockade at rest triggers rapid behavioral antidepressant responses. Nature. 2011;475:91–95.
Lucantonio F, Roeglin J, Li S, Lu J, Shi A, Czerpaniak K, et al. Ketamine rescues anhedonia by cell-type and input specific adaptations in the nucleus accumbens. Neuron. 2025;113:1398–412.
Castren E, Monteggia LM. Brain-derived neurotrophic factor signaling in depression and antidepressant action. Biol Psychiatry. 2021;90:128–36.
Wang CS, Kavalali ET, Monteggia LM. BDNF signaling in context: from synaptic regulation to psychiatric disorders. Cell. 2022;185:62–76.
Zarate CA Jr, Machado-Vieira R. Potential pathways involved in the rapid antidepressant effects of nitrous oxide. Biol Psychiatry. 2015;78:2–4.
Izumi Y, Zorumski CF. Metaplastic effects of subanesthetic ketamine on CA1 hippocampal function. Neuropharmacol. 2014;86:273–81.
Izumi Y, Hsu F-F, Conway CR, Nagele P, Mennerick SJ, Zorumski CF. Nitrous oxide, a rapid antidepressant, has ketamine-like effects on excitatory transmission in adult hippocampus. Biological Psychiatry. 2022;92:964–72.
Bevilacqua L, Charney A, Pierce CR, Richards SM, Jha MK, Glasgow A, et al. Role of nitric oxide signaling in the antidepressant mechanism of action of ketamine: A randomized controlled trial. J Psychopharmacol. 2021;35:124–7.
Kotani N, Jang I-S, Nakamura M, Nonaka K, Nagami H, Akaike N. Depression of synaptic NMDA responses by xenon and nitrous oxide. J Pharmacol Exp Therap. 2023;384:187–96.
Ranft A, Kurz J, Becker K, Dodt HU, Zieglgansberger W, Rammes G, et al. Nitrous oxide (N2O) pre- and postsynaptically attenuates NMDA receptor-mediated neurotransmission in the amygdala. Neuropharmacology. 2007;52:716–23.
Zanos P, Brown KA, Georgiou P, Yuan P, Zarate CA Jr, Thompson SM, et al. NMDA receptor activation-dependent antidepressant-relevant behavioral and synaptic actions of ketamine. J Neurosci. 2023;43:1038–50.
Kohtala S, Rantamaki T. Rapid-acting antidepressants and the regulation of TrkB neurotrophic signaling – insights from ketamine, nitrous oxide, seizures and anaesthesia. Basic Clin Pharmacol Toxicol. 2021;129:95–103.
Khlestova E, Johnson JW, Krystal JH, Lisman J. The role of GluN2C-containing NMDA receptors in ketamine’s psychotogenic action and in schizophrenia models. J Neurosci. 2016;36:11151–7.
Hanson JE, Yuan H, Perszyk RE, Banke TG, Xing H, Tsai M-C, et al. Therapeutic potential of N-methyl-D-aspartate receptor modulators in psychiatry. Neuropsychopharmacol. 2024;49:51–66.
Zorumski CF, Izumi Y, Mennerick S. Ketamine: NMDA receptors and beyond. J Neurosci. 2016;36:11158–66.
Krystal JH, Kavalali ET, Monteggia LM. Ketamine and rapid antidepressant action: new treatments and novel synaptic signaling mechanisms. Neuropsychopharmacol. 2024;49:41–50.
Nagashima K, Zorumski CF, Izumi Y. Nitrous oxide (laughing gas) facilitates excitability in rat hippocampal slices through γ-aminobutyric acid A receptor-mediated disinhibition. Anesthesiology. 2005;102:230–4.
Kohtala S, Theilmann W, Rosenholm M, Peena L, Karabulut G, Uusitalo S. Cortical excitability and activation of TrkB signaling during rebound slow oscillations are critical for rapid antidepressant responses. Mol Neurobiol. 2019;56:4163–74.
Alitalo O, Kohtala S, Rosenholm M, Saarreharju R, Gonzalez-Hernandez G, Sarparanta M, et al. Nitrous oxide induces hypothermia and TrkB activation: maintenance of body temperature abolishes antidepressant-like effects in mice. Neuropharmacol. 2024;261:110172.
Kohtala S, Parekh PK, Baramaki I, Kohtala P, Ilkka L, Antila H, et al. Nitrous oxide modulates cortical activity, wake-sleep oscillations, and produces antidepressant-like effects in mice. BioRxiv. [Preprint] 2025. https://doi.org/10.1101/2025.02.13.638096.
Valtonen P, Rozov S, Annala I, Jarventausta K, Subramaniyam NP, Tenhunen M, et al. Rebound electroencephalographic responses to nitrous oxide exposure in men. J Neurophysiol. 2025. https://doi.org/10.1152/jn.00275.2024.
Liu W, Li Q, Ye B, Cao H, Shen F, Xu Z, et al. Repeated nitrous oxide exposure exerts antidepressant-like effects through neuronal nitric oxide synthase activation in the medial prefrontal cortex. Front Psychiatry. 2020;11:837. https://doi.org/10.3389/fpsyt.2020.00837.
Li S, Ohgami Y, Dai Y, Quock RM. Antagonism of nitrous oxide-induced anxiolytic-like behavior in the mouse light/dark exploration procedure by pharmacologic disruption of endogenous nitric oxide function. Psychopharmacol. 2003;166:366–72.
Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–52.
Cichon J, Joseph TT, Lu X, Wasilczuk AZ, Kelz MB, Mennerick SJ, et al. Nitrous oxide activates layer 5 prefrontal neurons via SK2 channel inhibition for antidepressant effect. Nat Commun. 2025;16:2999. https://doi.org/10.1038/s41467-025-57951-y.
Abel HJ, Lee JCF, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarization in neocortical pyramidal neurons. J Neurophysiol. 2004;91:324–35.
Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, et al. Small conductance Ca2+ -activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24:5301–6.
Guan D, Armstrong WE, Foehring RC. Electrophysiological properties of genetically identified subtypes of layer 5 neocortical pyramidal neurons: Ca2+ dependence and differential modulation by norepinephrine. J Neurophysiol. 2015;113:2014–32.
Jiang Y, Dong Y, Hu H. The N-methyl-D-aspartate receptor hypothesis of ketamine’s antidepressant action: evidence and controversies. Phil Trans R Soc B. 2024;379:20230225.
Rozov S, Saarreharju R, Khirug S, Storvik M, Rivera C, Rantamaki T. Effects of nitrous oxide and ketamine on electrophysiological and molecular responses in the prefrontal cortex of mice: a comparative study. Eur J Pharmacol. 2024;968:176426. https://doi.org/10.1016/j.ejphar.2024.176426.
Brito MA, Li D, Fields CW, Rybicki-Kler C, Dean JG, Liu T, et al. Cortical acetylcholine levels correlate with neurophysiological complexity during subanesthetic ketamine and nitrous oxide exposure in rats. Anesth Analg. 2022;134:1126–39.
Li W-Q, Liu S-N, Yang S-C, Lin X, Zhang Z-J. Nitrous oxide exerts rewarding effect via regulating D1 receptor and BDNF pathway in ventral tegmental-nucleus accumbens dopamine circuit. Transl Psychiatry. 2025;15:34. https://doi.org/10.1038/s41398-025-03257-y.
Gillman MA. What is better for psychiatry: titrated or fixed concentrations of nitrous oxide? Front Psychiatry. 2022;13:773190. https://doi.org/10.3389/fpsyt.2022.773190.
Wolfgang AS, Fonzo GA, Gray JC, Krystal JH, Grzenda A, Widge AS, et al. MDMA and MDMA-assisted therapy. Am J Psychiatry. 2025;182:79–103.
Fonzo GA, Wolfgang AS, Barksdale BR, Krystal JH, Carpenter LL, Kraguljac NV, et al. Psilocybin: from psychiatric pariah to perceived panacea. Am J Psychiatry. 2025;182:54–78.
Goodwin GM, Malievskaia E, Fonzo GA, Nemeroff CB. Must psilocybin always “assist psychotherapy”? Am J Psychiatry. 2024;181:20–25.
Ma ZZ, Guzikowski NJ, Kim J-W, Kavalali ET, Monteggia LM. Enhanced ERK activity extends ketamine’s antidepressant effects by augmenting synaptic plasticity. Science. 2025;388:646–55.
De Leon V, Kumar A, Nagele P, Palanca BJ, Gott B, Janski A, et al. Nitrous oxide reduced suicidal ideation in treatment-resistant major depression in exploratory analysis. J Clin Psychiatry. 2023;84:22br14725.
Varias A, van Roessel P, Parsiani M, Filippou-Frye M, Neylan TC, Nagele P, et al. Does nitrous oxide help veterans with posttraumatic stress disorder? J Clin Psychiatry. 2020;81:20113383.
Das RK, Tamman A, Nikolova V, Freeman TP, Bisby JA, Lazzarino AI, et al. Nitrous oxide speeds the reduction of distressing intrusive memories in an experimental model of psychological trauma. Psychol Med. 2016;46:1749–59.
Williams E, Taujanskaite U, Kamboj SK, Murphy SE, Harmer CJ. Examining memory reconsolidation as a mechanism of nitrous oxide’s antidepressant action. Neuropsychopharmacol. 2025;50:609–17.
Das RK, Walsh K, Hannaford J, Lazzarino AI, Kamboj SK. Nitrous oxide may interfere with the reconsolidation of drinking memories in hazardous drinkers in a prediction-error-dependent manner. Eur J Neuropsychopharmacol. 2018;28:828–40.
Caton PW, Tousman SA, Quock RM. Involvement of nitric oxide in nitrous oxide anxiolysis in the elevated plus maze. Pharmacol Biochem Behav. 1994;48:689–92.
Emmanouil DE, Johnson CH, Quock RM. Nitrous oxide anxiolytic effect in mice in the elevated plus maze: mediation by benzodiazepine receptors. Psychopharmacol. 1994;115:167–72.
Emmanouil DE, Papadopoulou-Daifoti Z, Hagihara PT, Quock DG, Quock RM. A study of the role of serotonin in the anxiolytic effect of nitrous oxide in rodents. Pharmacol Biochem Behav. 2006;84:313–20.
Bartels P, Behnke K, Michels G, Groner F, Schneider T, Henry M, et al. Structural and biophysical determinants of single Ca(v)3.1 and Ca(v)3.2 T-type calcium channel inhibition by N(2)O. Cell Calcium. 2009;46:293–302.
Orestes P, Bojadzic D, Lee J, Leach E, Salajegheh R, Digruccio MR, et al. Free radical signaling underlies inhibition of Cav3.2 T-type calcium channels by nitrous oxide in the pain pathway. J Physiology. 2011;589:135–48.
Choi Y-B, Lipton SA. Redox modulation of the NMDA receptor. Cell Mol Life Sci. 2000;57:1535–41.
Chen M, Ma S, Liu H, Dong Y, Tang J, Ni Z, et al. Brain region-specific action of ketamine as a rapid antidepressant. Science. 2024;385:eado7010.
Chamaa F, Bahmad HF, Makkawi A-K, Chalhoub RM, Al-Chaer ED, Bikhazi GB, et al. Nitrous oxide induces prominent cell proliferation in adult rat hippocampal dentate gyrus. Front Cell Neurosci. 2018;12:135. https://doi.org/10.3389/fncel2018.00135.
Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C, et al. Ketamine treatment and global brain connectivity in major depression. Neuropsychopharmacol. 2017;42:1210–9.
Holmes DE, Finnema S, Naganawa M, DellaGioia N, Holden D, Fowles K, et al. Imaging the effect of ketamine on synaptic density (SV2A) in the living brain. Mol Psychiatry. 2022;27:2273–81.
Nugent AC, Wills KE, Gilbert JR, Zarate CA Jr. Synaptic potentiation and rapid antidepressant responses to ketamine in treatment-resistant major depression: a replication study. Psychiatry Res Neuroimag. 2019;283:64–66.
Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science. 2019;364:eaat88078.
Cichon J, Wasilczuk AZ, Looger LL, Contreras D, Kelz MB, Proekt A. Ketamine triggers a switch in excitatory neuronal activity across neocortex. Nature Neurosci. 2022;26:39–52.
Laumann TO, Zorumski CF, Dosenbach NUF. Precision neuroimaging for localization-related psychiatry. JAMA Psychiatry. 2023;80:763–4.
Lynch CJ, Elbau I, Liston C. Improving precision functional mapping routines with multi-echo fMRI. Curr Opin Behav Sci. 2021;40:113–9.
Fitzgerald PJ, Watson BO. Gamma oscillations as a biomarker for major depression: an emerging topic. Transl Psychiatry. 2018;8:177. https://doi.org/10.1038/s41398-018-0239-y.
Lambert PM, Ni R, Benz A, Rensing NR, Wong M, Zorumski CF, et al. Non-sedative cortical EEG signatures of allopregnanolone and functional comparators. Neuropsychopharmacol. 2023;48:371–9.
Davilia DG, McKinstry-Wu A, Kelz MB, Proekt A. The administration of ketamine is associated with dose-dependent stabilization of cortical dynamics in humans. J Neurosci. 2025;45:e1545242025.
Willsey MS, Nu CS, Nason SR, Schroeder KE, Hutchison BC, Welle EJ, et al. Neural dynamics in primate cortex during exposure to subanesthetic concentrations of nitrous oxide. eNeuro. 2021;14:8. ENEURO.0479-20.2021
Brenna CTA, Goldstein BI, Zarate Jr CA, Orser BA Repurposing general anesthetics drugs to treat depression: a new frontier for anesthesiologists in neuropsychiatric care. Anesthesiology. 2024. https://doi.org/10.1097/ALN.05037.
Tateiwa H, Evers AS. Neurosteroids and their potential as a safer class of general anesthetics. J Anesthesia. 2024;38:261–74.
Zorumski CF, Paul SM, Covey DF, Mennerick S. Neurosteroids as novel antidepressants and anxiolytics: GABA-A receptors and beyond. Neurobiol Stress. 2019;11:100196. https://doi.org/10.1016/j.ynstr.2019.10096.
Zorumski CF, Covey DF, Izumi Y, Evers AS, Maguire JL, Mennerick SJ New directions in neurosteroid therapeutics in neuropsychiatry. Neurosci Biobehav Rev. 2025. https://doi.org/10.1016/j.neubiorev.2025.106119.
Nagele P, Conway C, Zorumski CF Purposeful drug repurposing. JAMA Psychiatry. 2025. https://doi.org/10.1001/jamapsychiatry.2025.0900.
Zaki N, Chen LN, Lane R, Doherty T, Drevets WC, Morrison RL, et al. Safety and efficacy with esketamine in treatment-resistant depression: long-term extension study. Int J Neuropsychopharmacol. 2025;28:pyaf027. https://doi.org/10.1093/ijnp/pyaf027.
Myles F, Kulkarni J, Kasza J, Wallace S, Deng C, Turbic A, et al. Antidepressant effects of nitrous oxide in major depressive disorder: a phase 2b randomized clinical trial. Biological Psychiatry Glb Op Sci. 2025. https://doi.org/10.1016/j.bpsgos.2025.100504.
Acknowledgements
Work in the authors’ labs is supported by MH101874 (SM, CFZ), MH123748 (SM), MH122379 (CFZ, SM), MH108901 (CRC, PN), R35GM151160-01 (JC), the Taylor Family Institute for Innovative Psychiatric Research (SM, YI, CFZ, CRC), the Brain and Behavior Research Foundation (CRC, PN, JC), the American Foundation for Suicide Prevention (CRC, PN), and the Bantly Foundation (CFZ). The authors thank members of the Taylor Family Institute for helpful comments.
Author information
Authors and Affiliations
Contributions
CFZ conceptualized the paper and wrote the first draft of the manuscript. All other authors (JC, YI, TZ, SM, PN and CRC) contributed to the concepts and content of the article and to editing and approving the final draft. TZ also designed Fig. 1 and JC designed Fig. 2.
Corresponding author
Ethics declarations
Competing interests
CFZ served on the Scientific Advisory Board of Sage Therapeutics and had equity in the company. Sage Therapeutics did not fund or participate in this work. PN is a co-founder of NitroTherapeutics, Inc., which aims to develop nitrous oxide as a treatment for mood disorders. Other authors have no conflicts of interest to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zorumski, C.F., Cichon, J., Izumi, Y. et al. Rapid antidepressant potential of nitrous oxide: current state and major questions. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03439-6
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03439-6