Abstract
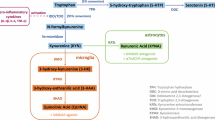
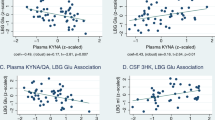
Abnormalities in the kynurenine pathway (KP) of tryptophan degradation, leading to the dysfunction of neuroactive KP metabolites in the brain, have been implicated in the pathophysiology of schizophrenia (SZ). One plausible mechanism involves dysregulation of various pro-inflammatory cytokines associated with the disease, which affect indoleamine-2,3-dioxygenase (IDO), a key enzyme for tryptophan to kynurenine conversion. In order to test this hypothesis directly, we measured plasma levels of the major KP metabolites kynurenine and kynurenic acid (KYNA), as well as four major cytokines, in a sample of 106 SZ patients and 104 control participants. In contrast to the replicable findings of elevation of KYNA in the central nervous system in SZ, plasma levels of KYNA were significantly lower in SZ compared to controls (p = .004). Kynurenine levels were significantly correlated with levels of interferon-γ (p < .001), which is involved in the regulation of IDO, in both patients and controls. However, although patients had higher levels of interleukin-6 (IL-6) compared to controls (p = .012), IL-6 levels were not correlated with kynurenine or KYNA, and did not explain group differences in KYNA. Based on the lack of evidence that pro-inflammatory cytokines were significantly related to the KP abnormality in SZ despite an adequate sample size, further studies must consider alternative hypotheses to identify the origins of the KP abnormalities in SZ.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77.
Kozak R, Campbell BM, Strick CA, Horner W, Hoffmann WE, Kiss T, et al. Reduction of brain kynurenic acid improves cognitive function. J Neurosci. 2014;34:10592–602.
Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophr Bull. 2010;36:211–8.
Wu HQ, Okuyama M, Kajii Y, Pocivavsek A, Bruno JP, Schwarcz R. Targeting kynurenine aminotransferase II in psychiatric diseases: promising effects of an orally active enzyme inhibitor. Schizophr Bull. 2014;40(Suppl 2):S152–8.
Miller CL, Llenos IC, Dulay JR, Barillo MM, Yolken RH, Weis S. Expression of the kynurenine pathway enzyme tryptophan 2,3-dioxygenase is increased in the frontal cortex of individuals with schizophrenia. Neurobiol Dis. 2004;15:618–29.
Miller CL, Llenos IC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulate cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073-1074:25–37.
Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–56.
Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–30.
Erhardt S, Blennow K, Nordin C, Skogh E, Lindström LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–8.
Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindström LH, et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res. 2005;80:315–22.
Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull. 2012;38:426–32.
Barry S, Clarke G, Scully P, Dinan TG. Kynurenine pathway in psychosis: evidence of increased tryptophan degradation. J Psychopharmacol. 2009;23:287–94.
Schwieler L, Larsson MK, Skogh E, Kegel ME, Orhan F, Abdelmoaty S, et al. Increased levels of IL-6 in the cerebrospinal fluid of patients with chronic schizophrenia–significance for activation of the kynurenine pathway. J Psychiatry Neurosci. 2015;40:126–33.
Chiappelli J, Postolache TT, Kochunov P, Rowland LM, Wijtenburg SA, Shukla DK, et al. Tryptophan metabolism and white matter integrity in schizophrenia. Neuropsychopharmacology. 2016;41:2587–95.
Plitman E, Iwata Y, Caravaggio F, Nakajima S, Chung JK, Gerretsen P, et al. Kynurenic acid in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2017;43:764–77.
Wurfel BE, Drevets WC, Bliss SA, McMillin JR, Suzuki H, Ford BN, et al. Serum kynurenic acid is reduced in affective psychosis. Transl Psychiatry. 2017;7:e1115.
Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139:655–62.
Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71.
Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–8.
Noto C, Ota VK, Gouvea ES, Rizzo LB, Spindola LM, Honda PH, et al. Effects of risperidone on cytokine profile in drug-naïve first-episode psychosis. Int J Neuropsychopharmacol. 2015;18:1–8.
Stojanovic A, Martorell L, Montalvo I, Ortega L, Monseny R, Vilella E, et al. Increased serum interleukin-6 levels in early stages of psychosis: associations with at-risk mental states and the severity of psychotic symptoms. Psychoneuroendocrinology. 2014;41:23–32.
Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155:101–8.
Wang AK, Miller BJ. Meta-analysis of cerebrospinal fluid cytokine and tryptophan catabolite alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder, and depression. Schizophr Bull. 2017;44:75–83.
Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, et al. The brief negative symptom scale: psychometric properties. Schizophr Bull. 2011;37:300–5.
Wechsler D. Wechsler Adult Intelligence Scale –Third Edition (WAIS-III). San Antonio, TX: Harcourt Assessment; 1997.
Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res. 2004;68:283–97.
Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–7.
Olsson SK, Larsson MK, Erhardt S. Subchronic elevation of brain kynurenic acid augments amphetamine-induced locomotor response in mice. J Neural Transm. 2012;119:155–63.
Pocivavsek A, Wu HQ, Elmer GI, Bruno JP, Schwarcz R. Pre- and postnatal exposure to kynurenine causes cognitive deficits in adulthood. Eur J Neurosci. 2012;35:1605–12.
Yeung D, Ciotti S, Purushothama S, Gharakhani E, Kuesters G, Schlain B, et al. Evaluation of highly sensitive immunoassay technologies for quantitative measurements of sub-pg/mL levels of cytokines in human serum. J Immunol Methods. 2016;437:53–63.
Capuron L, Schröcksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–82.
Quak J, Doornbos B, Roest AM, Duivis HE, Vogelzangs N, Nolen WA, et al. Does tryptophan degradation along the kynurenine pathway mediate the association between pro-inflammatory immune activity and depressive symptoms? Psychoneuroendocrinology. 2014;45:202–10.
Myint AM, Schwarz MJ, Verkerk R, Mueller HH, Zach J, Scharpé S, et al. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naïve and medication-free schizophrenic patients. Brain Behav Immun. 2011;25:1576–81.
Szymona K, Zdzisińska B, Karakuła-Juchnowicz H, Kocki T, Kandefer-Szerszeń M, Flis M, et al. Correlations of kynurenic acid, 3-hydroxykynurenine, sIL-2R, IFN-α, and IL-4 with clinical symptoms during acute relapse of schizophrenia. Neurotox Res. 2017;32:17–26.
Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–17.
Chiappelli J, Pocivavsek A, Nugent KL, Notarangelo FM, Kochunov P, Rowland LM, et al. Stress induced kynurenic acid as a potential biomarker for schizophrenia patients with distress intolerance. JAMA Psychiatry. 2014;71:761–8.
Schlittler M, Goiny M, Agudelo LZ, Venckunas T, Brazaitis M, Skurvydas A, et al. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. Am J Physiol Cell Physiol. 2016;310:C836–40.
Rassoulpour A, Wu HQ, Albuquerque EX, Schwarcz R. Prolonged nicotine administration results in biphasic, brain-specific changes in kynurenate levels in the rat. Neuropsychopharmacology. 2005;30:697–704.
Theofylaktopoulou D, Midttun Ø, Ulvik A, Ueland PM, Tell GS, Vollset SE, et al. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland Health Study. Clin Exp Immunol. 2013;173:121–30.
Holmes EW, Russell PM, Kinzler GJ, Bermes EW Jr.. Inflammation-associated changes in the cellular availability of tryptophan and kynurenine in renal transplant recipients. Clin Chim Acta. 1994;227:1–15.
Maciejak P, Szyndler J, Turzyńska D, Sobolewska A, Kołosowska K, Lehner M, et al. The kynurenine pathway: a missing piece in the puzzle of valproate action? Neuroscience. 2013;234:135–45.
Singh T, Goel RK. Adjuvant indoleamine 2,3-dioxygenase enzyme inhibition for comprehensive management of epilepsy and comorbid depression. Eur J Pharmacol. 2016;784:111–20.
Alexander KS, Wu HQ, Schwarcz R, Bruno JP. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacol. 2012;220:627–37.
Urata Y, Koga K, Hirota Y, Akiyama I, Izumi G, Takamura M, et al. IL-1β increases expression of tryptophan 2,3-dioxygenase and stimulates tryptophan catabolism in endometrioma stromal cells. Am J Reprod Immunol. 2014;72:496–503.
Joaquim HPG, Costa AC, Gattaz WF, Talib LL. Kynurenine is correlated with IL-1β in plasma of schizophrenia patients. J Neural Transm. 2018;6:1–5.
Kiank C, Zeden JP, Drude S, Domanska G, Fusch G, Otten W, et al. Psychological stress-induced, IDO1-dependent tryptophan catabolism: implications on immunosuppression in mice and humans. PLoS ONE. 2010;5:e11825.
Lavebratt C, Olsson S, Backlund L, Frisen L, Sellgren C, Priebe L, et al. The KMO allele encoding Arg is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol Psychiatry. 2014;19:334–41.
Na KS, Jung HY, Kim YK. The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:277–86.
Wonodi I, Stine OC, Sathyasaikumar KV, Roberts RC, Mitchell BD, Hong LE, et al. Downregulated kynurenine 3-monooxygenase gene expression and enzyme activity in schizophrenia and genetic association with schizophrenia endophenotypes. Arch Gen Psychiatry. 2011;68:665–74.
Acknowledgements
Funding
Funding support for this work was received from National Institutes of Health (grant numbers K23MH112010, P50 MH103222, U01MH108148, R01MH112180, R01MH085646, R01DA027680, R01MH094520, R01MH096263, T32MH067533).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. LEH has received or anticipates receiving research funding or consulting fees from Mitsubishi, Your Energy Systems LLC, Neuralstem, Taisho Pharmaceutical, Sound Pharma, Heptares, Pfizer, and Takeda. All remaining authors declare that they have no conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chiappelli, J., Notarangelo, F.M., Pocivavsek, A. et al. Influence of plasma cytokines on kynurenine and kynurenic acid in schizophrenia. Neuropsychopharmacol 43, 1675–1680 (2018). https://doi.org/10.1038/s41386-018-0038-4
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-018-0038-4
This article is cited by
-
Production, Mechanisms, and Therapeutic Strategies of Tryptophan Metabolites in CNS Diseases
Molecular Neurobiology (2026)
-
Elevated serum kynurenic acid in individuals with first-episode psychosis and insufficient response to antipsychotics
Schizophrenia (2024)
-
Importance of the dysregulation of the kynurenine pathway on cognition in schizophrenia: a systematic review of clinical studies
European Archives of Psychiatry and Clinical Neuroscience (2023)
-
The tryptophan catabolite or kynurenine pathway in schizophrenia: meta-analysis reveals dissociations between central, serum, and plasma compartments
Molecular Psychiatry (2022)
-
Kynurenine pathway in post-mortem prefrontal cortex and cerebellum in schizophrenia: relationship with monoamines and symptomatology
Journal of Neuroinflammation (2021)