Abstract
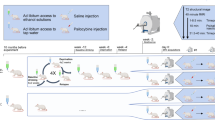
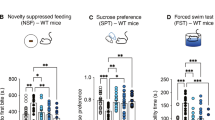
For most psychiatric disorders, including alcohol use disorder (AUD), approved pharmacological treatments are limited in their effectiveness, and new drugs that can easily be translated into the clinic are needed. Currently, great hope lies in the potential of psychedelics to effectively treat AUD. The primary hypothesis is that a single session of psychedelic-guided psychotherapy can restore normal brain function in AUD individuals and thereby reduce the risk of relapse in the long run. Here we applied three different treatment schedules with psilocybin/LSD in order to investigate relapse-like drinking in the alcohol deprivation effect (ADE) model. In contrast to the primary hypothesis, psychedelics had no long-lasting effects on the ADE in male and female rats, neither when administered in a high dosage regime that is comparable to the one used in clinical studies, nor in a chronic microdosing scheme. Only sub-chronic treatment with psilocybin produced a short-lasting anti-relapse effect. However, it is not a translatable treatment option to give psychedelics sub-chronically for relapse prevention. In conclusion, our results in the ADE model do not support the hypothesis that microdosing or high doses of psychedelic reduce relapse behavior. This conclusion has to be confirmed by applying other animal models of AUD. It could also well be that animal models of AUD might be unable to fully capture the therapeutic potential of psychedelic drugs and that only future large-scale clinical trials will be able to demonstrate the efficacy of psychedelics as a new treatment option for AUD.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kupferschmidt K. High hopes. Science. 2014;345:18–23.
dos Santos RG, Hallak JEC. Therapeutic use of serotoninergic hallucinogens: a review of the evidence and of the biological and psychological mechanisms. Neurosci Biobehav Rev. 2020;108:423–34.
Liechti ME. Modern clinical research on LSD. Neuropsychopharmacology 2017;42:2114–27.
Carhart-Harris RL, Goodwin GM. The therapeutic potential of psychedelic drugs: past, present, and future. Neuropsychopharmacology 2017;42:2105–13.
Hibicke M, Landry AN, Kramer HM, Talman ZK, Nichols CD. Psychedelics, but not ketamine, produce persistent antidepressant-like effects in a rodent experimental system for the study of depression. ACS Appl Mater Interfaces. 2020. https://doi.org/10.1021/acschemneuro.9b00493.
Polito V, Stevenson RJ. A systematic study of microdosing psychedelics. PLoS ONE. 2019;14:e0211023.
Anderson T, Petranker R, Rosenbaum D, Weissman CR, Dinh-Williams LA, Hui K, et al. Microdosing psychedelics: personality, mental health, and creativity differences in microdosers. Psychopharmacology. 2019;236:731–40.
Rosenbaum D, Weissman C, Anderson T, Petranker R, Dinh-Williams LA, Hui K, et al. Microdosing psychedelics: demographics, practices, and psychiatric comorbidities. J Psychopharmacol. 2020:269881120908004. https://doi.org/10.1177/0269881120908004 [Epub ahead of print].
Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the United States, 2001–2 to 2012–3: results from the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:911–23.
Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–33.
Spanagel R, Vengeliene V. New pharmacological treatment strategies for relapse prevention. Curr Top Behav Neurosci. 2012;13:583–609.
Fertig J, Leggio L, Litten RZ, Falk DE, Ryan ML. Advances in pharmacotherapy development: human clinical studies. Handbook of experimental pharmacology. Springer Nature, Switzerland. 2018;248:579–613.
Yardley MM, Ray LA. Medications development for the treatment of alcohol use disorder: insights into the predictive value of animal and human laboratory models. Addict Biol. 2017;22:581–615.
Bogenschutz MP. Studying the effects of classic hallucinogens in the treatment of alcoholism: rationale, methodology, and current research with psilocybin. Curr Drug Abus Rev. 2013;6:17–29.
Rucker JJH, Iliff J, Nutt DJ. Psychiatry & the psychedelic drugs. Past, present & future. Neuropharmacology. 2018;142:200–18.
Krebs TS, Johansen PØr. Lysergic acid diethylamide (LSD) for alcoholism: meta-analysis of randomized controlled trials. J Psychopharmacol. 2012;26:994–1002.
Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa P, Strassman RJ. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29:289–99.
Spanagel R. Animal models of addiction. Dialogues Clin Neurosci. 2017;19:247–58.
Vengeliene V, Bilbao A, Spanagel R. The alcohol deprivation effect model for studying relapse behavior: a comparison between rats and mice. Alcohol. 2014;48:313–20.
Vengeliene V, Celerier E, Chaskiel L, Penzo F, Spanagel R. Compulsive alcohol drinking in rodents. Addict Biol. 2009;14:384–96.
Hölter SM, Spanagel R. Effects of opiate antagonist treatment on the alcohol deprivation effect in long-term ethanol-experienced rats. Psychopharmacology. 1999;145:360–9.
Spanagel R, Hölter SM, Allingham K, Landgraf R, Zieglgänsberger W. Acamprosate and alcohol: I. Effects on alcohol intake following alcohol deprivation in the rat. Eur J Pharm. 1996;305:39–44.
Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. 2006;187:268–83.
Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology. 2011;218:649–65.
Cameron LP, Benson CJ, Dunlap LE, Olson DE. Effects of N, N-dimethyltryptamine on rat behaviors relevant to anxiety and depression. ACS Chem Neurosci. 2018;9:1582–90.
Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, et al. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018;23:3170–82.
Vengeliene V, Leonardi-Essmann F, Sommer WH, Marston HM, Spanagel R. Glycine transporter-1 blockade leads to persistently reduced relapse-like alcohol drinking in rats. Biol Psychiatry. 2010;68:704–11.
Ezquer F, Quintanilla ME, Morales P, Santapau D, Ezquer M, Kogan MJ, et al. Intranasal delivery of mesenchymal stem cell-derived exosomes reduces oxidative stress and markedly inhibits ethanol consumption and post-deprivation relapse drinking. Addict Biol. 2018;24:994–1007.
Sanchis-Segura C, Becker JB. Why we should consider sex (and study sex differences) in addiction research. Addict Biol. 2016;21:995–1006.
Vengeliene V, Bachteler D, Danysz W, Spanagel R. The role of the NMDA receptor in alcohol relapse: a pharmacological mapping study using the alcohol deprivation effect. Neuropharmacology. 2005;48:822–9.
Hölter SM, Engelmann M, Kirschke C, Liebsch G, Landgraf R, Spanagel R. Long-term ethanol self-administration with repeated ethanol deprivation episodes changes ethanol drinking pattern and increases anxiety-related behaviour during ethanol deprivation in rats. Behav Pharmacol. 1998;9:41–48.
Hölter SM, Henniger MSH, Lipkowski AW, Spanagel R. Kappa-opioid receptors and relapse-like drinking in long-term ethanol-experienced rats. Psychopharmacology. 2000;153:93–102.
Vengeliene V, Moeller A, Meinhardt MW, Beardsley PM, Sommer WH, Spanagel R, et al. The calpain inhibitor A-705253 attenuates alcohol-seeking and relapse with low side-effect profile. Neuropsychopharmacology. 2016;41:979–88.
Cameron LP, Benson CJ, Defelice BC, Fiehn O, Olson DE. Chronic, intermittent microdoses of the psychedelic N, N-dimethyltryptamine (DMT) produce positive effects on mood and anxiety in rodents. ACS Chem Neurosci. 2019;10:3261–70.
Davis M, Walters JK. Psilocybin: Biphasic dose-response effects on the acoustic startle reflex in the rat. Pharm Biochem Behav. 1977;6:427–31.
Jefsen O, Højgaard K, Christiansen SL, Elfving B, Nutt DJ, Wegener G, et al. Psilocybin lacks antidepressant-like effect in the Flinders Sensitive Line rat. Acta Neuropsychiatr. 2019. https://doi.org/10.1017/neu.2019.15.
Koerner J, Appel JB. Psilocybin as a discriminative stimulus: lack of specificity in an animal behavior model for ‘hallucinogens’. Psychopharmacology. 1982;76:130–5.
Johnstad PG. Powerful substances in tiny amounts: an interview study of psychedelic microdosing. NAD Nord Stud Alcohol Drugs. 2018;35:39–51.
Alper K, Dong B, Shah R, Sershen H, Vinod KY. LSD administered as a single dose reduces alcohol consumption in C57BL/6J mice. Front Pharmacol. 2018;9:994.
Lea T, Amada N, Jungaberle H. Psychedelic microdosing: a subreddit analysis. J Psychoactive Drugs. 2019. https://doi.org/10.1080/02791072.2019.1683260.
Lea T, Amada N, Jungaberle H, Schecke H, Klein M. Microdosing psychedelics: motivations, subjective effects and harm reduction. Int J Drug Policy. 2020;75:102600.
Horsley RR, Pálenícek T, Kolin J, Valeš K. Psilocin and ketamine microdosing: effects of subchronic intermittent microdoses in the elevated plus-maze in male Wistar rats. Behav Pharmacol. 2018;29:530–6.
Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705.
Celada P, Victoria Puig M, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABAA, and glutamate receptors. J Neurosci. 2001;21:9917–29.
Puig MV, Celada P, Díaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–82.
Vázquez-Borsetti P, Cortés R, Artigas F. Pyramidal neurons in rat prefrontal cortex projecting to ventral tegmental area and dorsal raphe nucleus express 5-HT2A receptors. Cereb Cortex. 2009;19:1678–86.
Vollenweider FX, Vontobel P, Hell D, Leenders KL. 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in Man—a PET study with [11C] raclopride. Neuropsychopharmacology. 1999;20:424–33.
González-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, et al. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–52.
Poirier R, Cheval H, Mailhes C, Charnay P, Davis S, Laroche S. Paradoxical role of an Egr transcription factor family member, Egr2/Krox20, in learning and memory. Front Behav Neurosci. 2007;1:6.
Meinhardt MW, Hansson AC, Perreau-Lenz S, Bauder-Wenz C, Stählin O, Heilig M, et al. Rescue of infralimbic mGluR2 deficit restores control over drug-seeking behavior in alcohol dependence. J Neurosci. 2013;33:2794–806.
Smart RG, Storm T, Baker EF, Solursh L. A controlled study of lysergide in the treatment of alcoholism. 1. The effects on drinking behavior. Q J Stud Alcohol. 1966;27:469–82.
Meinhardt MW, Sommer WH. Postdependent state in rats as a model for medication development in alcoholism. Addict Biol. 2015;20:1–21.
Bell RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–88.
Heinz A, Kiefer F, Smolka MN, Endrass T, Beste C, Beck A, et al. Addiction Research Consortium: Losing and regaining control over drug intake (ReCoDe)—from trajectories to mechanisms and interventions. Addict Biol. 2020;25:e12866.
Funding and disclosure
Financial support for this work was provided by the Bundesministerium für Bildung und Forschung (BMBF) funded ERA-NET program: Psi-Alc (FKZ: 01EW1908), the BMBF-funded SysMedSUDs consortium (FKZ: 01ZX1909A), and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project-ID 402170461—TRR 265 [54]. The authors declare no competing financial interests.
Author information
Authors and Affiliations
Contributions
MWM, CG, and RS designed research; MWM, CG, IS, and LJM performed research; MWM, CG, IS, and LJM analyzed data; MWM, IS, LJM, and RS wrote the paper.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Meinhardt, M.W., Güngör, C., Skorodumov, I. et al. Psilocybin and LSD have no long-lasting effects in an animal model of alcohol relapse. Neuropsychopharmacol. 45, 1316–1322 (2020). https://doi.org/10.1038/s41386-020-0694-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-0694-z
This article is cited by
-
Letter to the editor: Comments on ‘Striking long-term beneficial effects of single-dose psilocybin and psychedelic mushroom extract in the SAPAP3 rodent model of OCD-like excessive self-grooming’ by Brownstien et al. (2024)
Molecular Psychiatry (2025)
-
Exploring the therapeutic potential of psychedelics in treating substance use disorders
Molecular Psychiatry (2025)
-
Psilocybin reduces heroin seeking behavior and modulates inflammatory gene expression in the nucleus accumbens and prefrontal cortex of male rats
Molecular Psychiatry (2025)
-
Psilocybin and MDMA in Couples Therapy: Investigating Treatment for Substance Use Disorders and Codependency
Contemporary Family Therapy (2025)
-
Therapeutic mechanisms of psychedelics and entactogens
Neuropsychopharmacology (2024)