Abstract
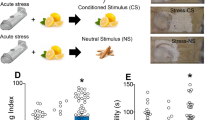
Even a single 2-hour episode of immobilization stress is known to trigger anxiety-like behavior and increase spine-density in the basolateral amygdala (BLA) of rats 10 days later. This delayed build-up of morphological and behavioral effects offers a stress-free time window of intervention after acute stress, which we used to test a protective role for glucocorticoids against stress. We observed that post-stress corticosterone, given 1 day after acute stress in drinking water, reversed enhanced anxiety-like behavior 10 days later. Quantification of spine-density on Golgi-stained BLA principal neurons showed that the same intervention also prevented the increase in spine numbers in the amygdala, at the same delayed time-point. Further, stress elevated serum corticosterone levels in rats that received vehicle in the drinking water. However, when stress was followed 24 h later by corticosterone in the drinking water, the surge in corticosterone was prevented. Together, these observations suggest that corticosterone, delivered through drinking water even 24 h after acute stress, is capable of reversing the delayed enhancing effects on BLA synaptic connectivity and anxiety-like behavior. Strikingly, although the immobilization-induced surge in corticosterone by itself has delayed detrimental effects on amygdalar structure and function, there exists a window of opportunity even after stress to mitigate its impact with a second surge of exogenously administered corticosterone. This provides a framework in the amygdala for analyzing how the initial physiological and endocrine processes triggered by traumatic stress eventually give rise to debilitating emotional symptoms, as well as the protective effects of glucocorticoids against their development.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chattarji AS, Tomar A, Suvrathan A, Ghosh S. Neighborhood matters: divergent patterns of stress - induced plasticity across the brain Affiliations. Nat Publ Gr. 2015;18:1–39.
McEwen BS, Nasca C, Gray JD. Stress effects on neuronal structure: hippocampus, amygdala and prefrontal cortex. Neuropsychopharmacology. 2016;41:3–23. https://doi.org/10.1038/npp.2015.171.
Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33.
Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci USA. 2005;102:9371–6.
Rao RP, Anilkumar S, McEwen BS, Chattarji S. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry. 2012;72:466–75.
Yasmin F, Saxena K, McEwen BS, Chattarji S. The delayed strengthening of synaptic connectivity in the amygdala depends on NMDA receptor activation during acute stress. Physiol Rep. 2016;4:1–11.
Chakraborty P, Chattarji S. Interventions after acute stress prevent its delayed effects on the amygdala. Neurobiol Stress. 2019;10:1–23.
Rao RP, Suvrathan A, Miller MM, McEwen BS, Chattarji S. PTSD: From Neurons to Networks. Neurobiol. PTSD. New York: Humana Press; 2009. p. 151–85.
Bryant RA. Post‐traumatic stress disorder: a state‐of‐the‐art review of evidence and challenges. World Psychiatry. 2019;18:259–69. https://doi.org/10.1002/wps.20656.
Schelling G, Kilger E, Roozendaal B, De Quervain DJF, Briegel J, Dagge A, et al. Stress doses of hydrocortisone, traumatic memories, and symptoms of posttraumatic stress disorder in patients after cardiac surgery: A randomized study. Biol Psychiatry. 2004;55:627–33.
Weis F, Kilger E, Roozendaal B, De Quervain DJF, Lamm P, Schmidt M, et al. Stress doses of hydrocortisone reduce chronic stress symptoms and improve health-related quality of life in high-risk patients after cardiac surgery: a randomized study. J Thorac Cardiovasc Surg. 2006;131:277–82.
Schelling G, Roozendaal B, Krauseneck T, Schmoelz M, De Quervain D, Briegel J. Efficacy of hydrocortisone in preventing posttraumatic stress disorder following critical illness and major surgery. Ann N Y Acad Sci. 2006;1071:46–53.
Schelling G, Briegel J, Roozendaal B, Stoll C, Rothenha H. The effect of stress doses of hydrocortisone during septic shock on posttraumatic stress disorder in survivors. Biol Psychiatry. 2001;50:978–85.
Zohar J, Yahalom H, Kozlovsky N, Cwikel-Hamzany S, Matar MA, Kaplan Z, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol. 2011;21:796–809.
Cohen H, Matar MA, Buskila D, Kaplan Z, Zohar J. Early post-stressor intervention with high-dose corticosterone attenuates posttraumatic stress response in an animal model of posttraumatic stress disorder. Biol Psychiatry. 2008;64:708–17.
Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, et al. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59:1208–18.
Collimore KC, Carleton RN, Hofmann SG, Asmundson GJG. Posttraumatic stress and social anxiety: the interaction of traumatic events and interpersonal fears. Depress Anxiety. 2010;27:1017–26.
Christianson JP, Thompson BM, Watkins LR, Maier SF. Medial prefrontal cortical activation modulates the impact of controllable and uncontrollable stressor exposure on a social exploration test of anxiety in the rat. Stress. 2009;12:445–50.
Varela JA, Wang J, Christianson JP, Maier SF, Cooper DC. Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability. J Neurosci. 2012;32:12848–53.
Chakraborty P, Chattarji S. Timing is everything: differential effects of chronic stress on fear extinction. Psychopharmacology (Berl). 2019;236:73–86. https://doi.org/10.1007/s00213-018-5053-y.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates: compact sixth edition. New York: Academic Press; 2009.
Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8.
Madan JS, Gupta K, Chattarji S, Bhattacharya A. Hippocampal and amygdalar cell-specific translation is similar soon after stress but diverge over time. Hippocampus. 2018;28:441–52.
Schelling G, Stoll C, Haller M, Kapfhammer HP, Rothenhausler HB, Krauseneck T, et al. The effect of hydrocortisone during septic shock on post-traumatic stress disorder (Ptsd) in survivors. Anesthesiology. 2003;89:429A.
Noto Y, Sato T, Kudo M, Kurata K, Hirota K. The relationship between salivary biomarkers and state-trait anxiety inventory score under mental arithmetic stress: a pilot study. Anesth Analg. 2005;101:1873–6.
Takai N, Yamaguchi M, Aragaki T, Eto K, Uchihashi K, Nishikawa Y. Effect of psychological stress on the salivary cortisol and amylase levels in healthy young adults. Arch Oral Biol. 2004;49:963–8.
Armario A, Marti O, Molina T, De Pablo J, Valdes M. Acute stress markers in humans: response of plasma glucose, cortisol and prolactin to two examinations differing in the anxiety they provoke. Psychoneuroendocrinology. 1996;21:17–24.
Aerni A, Traber R, Hock C, Roozendaal B, Schelling G, Papassotiropoulos A, et al. Low-dose cortisol for symptoms of posttraumatic stress disorder. Am J Psychiatry. 2004;161:1488–90.
Soravia LM, Heinrichs M, Aerni A, Maroni C, Schelling G, Ehlert U, et al. Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci. 2006;103:5585–90.
Yasmin F, Colangeli R, Morena M, Filipski S, van der Stelt M, Pittman QJ, et al. Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proc Natl Acad Sci. 2020;117:650–5.
Cohen S, Kozlovsky N, Matar MA, Kaplan Z, Zohar J, Cohen H. Post-exposure sleep deprivation facilitates correctly timed interactions between glucocorticoid and adrenergic systems, which attenuate traumatic stress responses. Neuropsychopharmacology. 2012;37:2388–404.
Zuckerman A, Ram O, Ifergane G, Matar MA, Kaplan Z, Hoffman JR, et al. Role of endogenous and exogenous corticosterone on behavioral and cognitive responses to low-pressure blast wave exposure. J Neurotrauma. 2018;36:380–94.
Jia M, Smerin SE, Zhang L, Xing G, Li X, Benedek D, et al. Corticosterone mitigates the stress response in an animal model of PTSD. J Psychiatr Res. 2015;60:29–39.
Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin North Am. 2013;42:503–13.
Yehuda R, Hoge CW, McFarlane AC, Vermetten E, Lanius RA, Nievergelt CM, et al. Post-traumatic stress disorder. Nat Rev Dis Prim. 2015;1:1–22.
Yehuda R, Bierer LM, Pratchett LC, Lehrner A, Koch EC, Manen JAVan, et al. Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology. 2015;51:589–97.
Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci. 2008;105:5573–8.
Kim H, Yi J, Choi K, Hong S, Shin K, Kang S. Regional differences in acute corticosterone-induced dendritic remodeling in the rat brain and their behavioral consequences. BMC Neurosci. 2014;15:65.
Liston, C, Gan, WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci. 2011;108:16074–9.
Rajkumar RP, Bharadwaj B. Pharmacological prevention of posttraumatic stress disorder: a systematic review. Adv Psychiatry. 2014;2014:10.
Ludäscher P, Schmahl C, Feldmann RE, Kleindienst N, Schneider M, Bohus M. No evidence for differential dose effects of hydrocortisone on intrusive memories in female patients with complex post-traumatic stress disorder – a randomized, double-blind, placebo-controlled, crossover study. J Psychopharmacol. 2015;29:1077–84.
Astill Wright L, Sijbrandij M, Sinnerton R, Lewis C, Roberts NP, Bisson JI. Pharmacological prevention and early treatment of post-traumatic stress disorder and acute stress disorder: a systematic review and meta-analysis. Transl Psychiatry. 2019;9:1–10.
Ashokan A, Sivasubramanian M, Mitra R. Seeding Stress Resilience through Inoculation. Neural Plast. 2016;1–6.
Brockhurst J, Cheleuitte-Nieves C, Buckmaster CL, Schatzberg AF, Lyons DM. Stress inoculation modeled in mice. Transl Psychiatry. 2015;5:1–5.
Lyons DM, Parker KJ, Katz M, Schatzberg AF. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front Behav Neurosci. 2009;3:1–6.
Hourani LL, Kizakevich PN, Hubal R, Spira J, Strange LB, Holiday DB, et al. Predeployment stress inoculation training for primary prevention of combat-related stress disorders. J Cybertherapy Rehabil. 2011;4:101–16.
Galatzer-Levy IR, Andero R, Sawamura T, Jovanovic T, Papini S, Ressler KJ, et al. A cross species study of heterogeneity in fear extinction learning in relation to FKBP5 variation and expression: Implications for the acute treatment of posttraumatic stress disorder. Neuropharmacology. 2017;116:188–95.
Sawamura T, Klengel T, Armario A, Jovanovic T, Norrholm SD, Ressler KJ, et al. Dexamethasone treatment leads to enhanced fear extinction and dynamic Fkbp5 regulation in amygdala. Neuropsychopharmacology. 2016;41:832–46.
Inoue R, Abdou K, Hayashi-Tanaka A, Muramatsu SI, Mino K, Inokuchi K, et al. Glucocorticoid receptor-mediated amygdalar metaplasticity underlies adaptive modulation of fear memory by stress. Elife. 2018;7:1–18.
Karst H, Berger S, Erdmann G, Schutz G, Joels M. Metaplasticity of amygdalar responses to the stress hormone corticosterone. Proc Natl Acad Sci. 2010;107:14449–54.
Joëls M, Sarabdjitsingh RA, Karst H. Unraveling the time domains of corticosteroid hormone influences on brain activity: rapid, slow, and chronic modes. Pharmacol Rev. 2012;64:901–38.
Acknowledgements
PC and SC would like to express their unending gratitude to Prof. Bruce McEwen for his generous advice and continued guidance during this project, from the early days of its conceptualization to its completion.
Author information
Authors and Affiliations
Contributions
PC, BM and SC contributed to the experimental design. PC and SD performed the experiments and analyzed the data. PC, BM and SC interpreted the results. PC and SC wrote the manuscript.
Corresponding author
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chakraborty, P., Datta, S., McEwen, B.S. et al. Corticosterone after acute stress prevents the delayed effects on the amygdala. Neuropsychopharmacol. 45, 2139–2146 (2020). https://doi.org/10.1038/s41386-020-0758-0
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-020-0758-0
This article is cited by
-
Combined Restraint Stress and Metal Exposure Paradigms in Rats: Unravelling Behavioural and Neurochemical Perturbations
Molecular Neurobiology (2025)
-
Corticosterone after early adolescent stress prevents social avoidance, aversive behavior, and morphine-conditioned place preference in adulthood
Psychopharmacology (2024)
-
Lateral hypothalamic proenkephalin neurons drive threat-induced overeating associated with a negative emotional state
Nature Communications (2023)
-
Accelerated forgetting of a trauma-like event in healthy men and women after a single dose of hydrocortisone
Translational Psychiatry (2022)
-
“Corting” stress: post-stress corticosterone administration prevents delayed-onset biobehavioral consequences
Neuropsychopharmacology (2020)