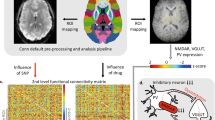
Abstract
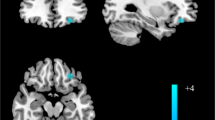
Altered striatal regulation of the GluN2B subunit of N-methyl-D-aspartate (NMDA) glutamate receptors by the Fyn/Src family of protein tyrosine kinases has been implicated in animal alcohol consumption. Previously, we have described differences between individuals positive (FHP) and negative (FHN) for familial alcohol use disorder (AUD) in the ventral striatal (VS) activation associated with monetary incentive delay task (MIDT) performance during functional magnetic resonance imaging (fMRI). Here, we used AZD0530 (saracatinib), a centrally active Fyn/Src inhibitor to probe the role of Fyn/Src regulation of NMDA receptors (NMDAR) in VS activation differences between FHP and FHN individuals during fMRI MIDT performance. We studied 21 FHN and 22 FHP individuals, all without AUD. In two sessions, spaced 1 week apart, we administered 125 mg of saracatinib or placebo in a double-blind manner, prior to measuring VS signal during fMRI MIDT performance. MIDT comprises reward prospect, anticipation, and outcome phases. During the initial (prospect of reward) task phase, there was a significant group-by-condition interaction such that, relative to placebo, saracatinib reduced VS BOLD signal in FHP and increased it in FHN individuals. This study provides the first human evidence that elevated signaling in striatal protein kinase A-dependent pathways may contribute to familial AUD risk via amplifying the neural response to the prospect of reward. As Fyn kinase is responsible for NMDAR upregulation, these data are consistent with previous evidence for upregulated NMDAR function within reward circuitry in AUD risk. These findings also suggest a possible therapeutic role for Src/Fyn kinase inhibitors in AUD risk.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kendler KS, Ohlsson H, Sundquist J, Sundquist K. Prediction of onset of substance-induced psychotic disorder and its progression to Schizophrenia in a Swedish national sample. Am J Psychiatry. 2019;176:711–9.
Richmond-Rakerd LS, Slutske WS, Lynskey MT, Agrawal A, Madden PA, Bucholz KK, et al. Age at first use and later substance use disorder: shared genetic and environmental pathways for nicotine, alcohol, and cannabis. J Abnorm Psychol. 2016;125:946–59.
Whipple SC, Berman SM, Noble EP. Event-related potentials in alcoholic fathers and their sons. Alcohol. 1991;8:321–7.
Finn PR, Earleywine M, Pihl RO. Sensation seeking, stress reactivity, and alcohol dampening discriminate the density of a family history of alcoholism. Alcohol Clin Exp Res. 1992;16:585–90.
Hesselbrock MN, Hesselbrock VM. Relationship of family history, antisocial personality disorder and personality traits in young men at risk for alcoholism. J Stud Alcohol. 1992;53:619–25.
Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 2002;63:83–90.
Saunders B, Farag N, Vincent AS, Collins FL Jr, Sorocco KH, Lovallo WR. Impulsive errors on a Go-NoGo reaction time task: disinhibitory traits in relation to a family history of alcoholism. Alcohol Clin Exp Res. 2008;32:888–94.
Mello NK, Mendelson JH. Behavioral studies of sleep patterns in alcoholics during intoxication and withdrawal. J Pharm Exp Ther. 1970;175:94–112.
Mello NK, Mendelson JH. Experimentally induced intoxication in alcoholics: a comparison between programed and spontaneous drinking. J Pharm Exp Ther. 1970;173:101–16.
Schuckit MA. A 10-year follow-up of sons of alcoholics: preliminary results. Alcohol Alcohol Suppl. 1991;1:147–9.
Narayanan B, Stevens MC, Jiantonio RE, Krystal JH, Pearlson GD. Effects of memantine on event-related potential, oscillations, and complexity in individuals with and without family histories of alcoholism. J Stud Alcohol Drugs. 2013;74:245–57.
Dickerson D, Pittman B, Ralevski E, Perrino A, Limoncelli D, Edgecombe J, et al. Ethanol-like effects of thiopental and ketamine in healthy humans. J Psychopharmacol. 2010;24:203–11.
Petrakis IL, Limoncelli D, Gueorguieva R, Jatlow P, Boutros NN, Trevisan L, et al. Altered NMDA glutamate receptor antagonist response in individuals with a family vulnerability to alcoholism. Am J Psychiatry. 2004;161:1776–82.
Walsh K, Das RK, Kamboj SK. The subjective response to nitrous oxide is a potential pharmaco-endophenotype for alcohol use disorder: a preliminary study with heavy drinkers. Int J Neuropsychopharmacol. 2017;20:346–50.
Jamadar S, DeVito EE, Jiantonio RE, Meda SA, Stevens MC, Potenza MN, et al. Memantine, an NMDA receptor antagonist, differentially influences Go/No-Go performance and fMRI activity in individuals with and without a family history of alcoholism. Psychopharmacol (Berl). 2012;222:129–40.
Holmes A, Spanagel R, Krystal JH. Glutamatergic targets for new alcohol medications. Psychopharmacol (Berl). 2013;229:539–54.
Robbins C. Sex differences in psychosocial consequences of alcohol and drug abuse. J Health Soc Behav. 1989;30:117–30.
Jentsch JD, Roth RH, Taylor JR. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res. 2000;126:433–53.
Wise RA, Koob GF. The development and maintenance of drug addiction. Neuropsychopharmacology. 2014;39:254–62.
Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–8.
Mao LM, Wang JQ. Dopamine D2 receptors are involved in the regulation of Fyn and metabotropic glutamate receptor 5 phosphorylation in the rat striatum in vivo. J Neurosci Res. 2016;94:329–38.
Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2012;279:12–9.
Dunah AW, Sirianni AC, Fienberg AA, Bastia E, Schwarzschild MA, Standaert DG. Dopamine D1-dependent trafficking of striatal N-methyl-D-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol Pharm. 2004;65:121–9.
Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G, et al. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28:720–8.
Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–41.
Thanos PK, Wang GJ, Volkow ND. Positron emission tomography as a tool for studying alcohol abuse. Alcohol Res Health. 2008;31:233–7.
Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, et al. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–8.
Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, et al. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–86.
Morisot N, Berger AL, Phamluong K, Cross A, Ron D. The Fyn kinase inhibitor, AZD0530, suppresses mouse alcohol self-administration and seeking. Addict Biol. 2019;24:1227–34.
Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003;27:1736–42.
Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–98.
Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, et al. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol Psychiatry. 2011;69:675–83.
Luijten M, Schellekens AF, Kuhn S, Machielse MW, Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA psychiatry. 2017;74:387–98.
Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–42.
Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, et al. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage 2007;35:787–94.
Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–76.
Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 2000;95:S91–117. Suppl 2.
Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 2001;12:3683–7.
Knutson B, Momenan R, Rawlings RR, Fong GW, Hommer D. Negative association of neuroticism with brain volume ratio in healthy humans. Biol Psychiatry. 2001;50:685–90.
Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP. et al. N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem.2006;49:6465–88.
Wechsler D (1999): Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation.
First MB, Williams, JB, Karg, RS, Spitzer, RL (2015): User’s Guide for the Structured Clinical Interview for DSM-5 Disorders, Research Version (SCID-5-RV). Arlington, VA: American Psychiatric Association.
Nygaard HB, Wagner AF, Bowen GS, Good SP, MacAvoy MG, Strittmatter KA, et al. A phase Ib multiple ascending dose study of the safety, tolerability, and central nervous system availability of AZD0530 (saracatinib) in Alzheimer’s disease. Alzheimers Res Ther. 2015;7:35.
Patel KT, Stevens MC, Meda SA, Muska C, Thomas AD, Potenza MN, et al. Robust changes in reward circuitry during reward loss in current and former cocaine users during performance of a monetary incentive delay task. Biol Psychiatry. 2013;74:529–37.
World Health O (2001): AUDIT: the Alcohol Use Disorders Identification Test: guidelines for use in primary health care/Thomas F. Babor, [et al.]. 2nd ed ed. Geneva: World Health Organization.
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27.
Miller WR, Tonigan, JS and Longbaugh, R (1995): The Drinker Inventory of Consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse (Test Manual). Bethesda, MD: Department of Health and Human Services, pp 95–3911.
Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74.
Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol. 1994;67:319–33.
Brett M, Anton JL, Valabregue R, Poline JB (2002): Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain. Sendai, Japan: Neuroimage.
Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:48.
Wickham H (2016): ggplot2: Elegant graphics for data analysis. New York: Springer-Verlang.
Hill SY, Sharma VK. DRD2 methylation and regional grey matter volumes in young adult offspring from families at ultra-high risk for alcohol dependence. Psychiatry Res Neuroimaging. 2019;286:31–8.
Alvanzo AA, Wand GS, Kuwabara H, Wong DF, Xu X, McCaul ME. Family history of alcoholism is related to increased D2 /D3 receptor binding potential: a marker of resilience or risk? Addict Biol. 2017;22:218–28.
Kegeles LS, Horga G, Ghazzaoui R, Rosengard R, Ojeil N, Xu X, et al. Enhanced striatal dopamine release to expectation of alcohol: a potential risk factor for alcohol use disorder. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:591–8.
Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, et al. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–24.
Krishnan-Sarin S, O’Malley SS, Franco N, Cavallo DA, Morean M, Shi J, et al. N-methyl-D-aspartate receptor antagonism has differential effects on alcohol craving and drinking in heavy drinkers. Alcohol Clin Exp Res. 2015;39:300–7.
Krupitsky EM, Neznanova O, Masalov D, Burakov AM, Didenko T, Romanova T, et al. Effect of memantine on cue-induced alcohol craving in recovering alcohol-dependent patients. Am J Psychiatry. 2007;164:519–23.
Evans SM, Levin FR, Brooks DJ, Garawi F. A pilot double-blind treatment trial of memantine for alcohol dependence. Alcohol Clin Exp Res. 2007;31:775–82.
Yau WY, Zubieta JK, Weiland BJ, Samudra PG, Zucker RA, Heitzeg MM. Nucleus accumbens response to incentive stimuli anticipation in children of alcoholics: relationships with precursive behavioral risk and lifetime alcohol use. J Neurosci. 2012;32:2544–51.
Dhingra I, Zhang S, Zhornitsky S, Le TM, Wang W, Chao HH, et al. The effects of age on reward magnitude processing in the monetary incentive delay task. Neuroimage. 2020;207:116368.
Funding information
This study was funded by 2P50 AA012870 to JK. Study drug and placebo were provided by Astra-Zeneca. Dr. Potenza has consulted for and advised Game Day Data, the Addiction Policy Forum, AXA, Idorsia, and Opiant Therapeutics; has received research support from the Connecticut Council on Problem Gambling, Mohegan Sun Casino, and the National Center for Responsible Gaming (now the International Center for Responsible Gambling); has participated in surveys, mailings, or telephone consultations related to addictions, impulse-control disorders or other health topics; has consulted for law offices and the federal public defender’s office in issues related to impulse-control and addictive disorders; has provided clinical care in the Connecticut Department of Mental Health and Addiction Services Problem Gambling Services Program; has performed grant reviews for the National Institutes of Health and other agencies; has edited journals and journal sections; has given academic lectures in grand rounds, CME events and other clinical/scientific venues; and has generated books or chapters for publishers of mental health texts. All other authors report no biomedical financial interests or potential conflicts of interest.
Author information
Authors and Affiliations
Contributions
KTP, GDP, MCS, SOM MNP & JHK all contributed to manuscript authorship. KTP performed statistical analyses. AD and AG were responsible for subject recruitment and data collection. KDM was responsible for administrative aspects of the study. All authors reviewed & commented on the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Patel, K.T., Stevens, M.C., Dunlap, A. et al. Effects of the Fyn kinase inhibitor saracatinib on ventral striatal activity during performance of an fMRI monetary incentive delay task in individuals family history positive or negative for alcohol use disorder. A pilot randomised trial. Neuropsychopharmacol. 47, 840–846 (2022). https://doi.org/10.1038/s41386-021-01157-5
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-021-01157-5