Abstract
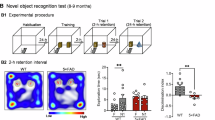
Alzheimer’s disease (AD) is the most common form of dementia with no effective treatment options. A complete elucidation of its underlying molecular mechanisms, including the transcription regulation of genes critically involved in AD, may shed light on new therapeutic development. RPS23RG1 is a newly identified AD-associated gene, whose expression is decreased in AD and restoration can attenuate AD-like phenotypes in animal models. However, the transcription regulation of RPS23RG1 remains unknown. In this study, we explored the promoter of RPS23RG1 and identified its transcription initiation site (TSS) at 1525 bp upstream of the ATG translation start codon. Progressive deletion analysis determined the presence of a negative regulatory region and a positive regulatory region within nucleotide positions +1127 to +1187 and +732 to +1127 relative to the TSS (+1), respectively. We conducted a reporter system to screen for compounds that increase RPS23RG1 expression through antagonizing its negative regulatory elements and identified phenazopyridine. Importantly, we demonstrated that phenazopyridine not only promoted RPS23RG1/Rps23rg1 expression, but also reduced AD-like pathologies and cognitive impairments in the APP/PS1 AD model mice. We also determined a critical negative regulatory domain of RPS23RG1 within nucleotide positions +1177 to +1187 and found that the transcription factor SMAD3 bound to this domain. Inhibition of SMAD3 promoted RPS23RG1 expression. Moreover, phenazopyridine reduced SMAD3 binding to the RPS23RG1 promoter without affecting SMAD3 phosphorylation and nuclear localization. Taken together, our results determine the transcription regulation mechanism of RPS23RG1 and show that phenazopyridine has potential for AD treatment through regulating RPS23RG1 transcription.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Rahman MM, Lendel C. Extracellular protein components of amyloid plaques and their roles in Alzheimer’s disease pathology. Mol Neurodegener. 2021;16:59.
Hanseeuw BJ, Betensky RA, Jacobs HIL, Schultz AP, Sepulcre J, Becker JA, et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. JAMA Neurol. 2019;76:915–24.
Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener. 2020;15:40.
O’Brien RJ, Wong PC. Amyloid precursor protein processing and Alzheimer’s disease. Annu Rev Neurosci. 2011;34:185–204.
Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42.
Dahlgren KN, Manelli AM, Stine WB Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-beta peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–53.
Xia Y, Prokop S, Giasson BI. “Don’t Phos Over Tau”: recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer’s disease and other tauopathies. Mol Neurodegener. 2021;16:37.
Seto M, Weiner RL, Dumitrescu L, Hohman TJ. Protective genes and pathways in Alzheimer’s disease: moving towards precision interventions. Mol Neurodegener. 2021;16:29.
2020 Alzheimer’s disease facts and figures. Alzheimers Dement. 2020;16:391–460.
McDade E, Llibre-Guerra JJ, Holtzman DM, Morris JC, Bateman RJ. The informed road map to prevention of Alzheimer disease: a call to arms. Mol Neurodegener. 2021;16:49.
Zhang YW, Liu S, Zhang X, Li WB, Chen Y, Huang X, et al. A functional mouse retroposed gene Rps23r1 reduces Alzheimer’s beta-amyloid levels and tau phosphorylation. Neuron. 2009;64:328–40.
Zhao D, Meng J, Zhao Y, Huo Y, Liu Y, Zheng N, et al. RPS23RG1 is required for synaptic integrity and rescues Alzheimer’s disease-associated cognitive deficits. Biol Psychiatry. 2019;86:171–84.
Huang X, Chen Y, Li WB, Cohen SN, Liao FF, Li L, et al. The Rps23rg gene family originated through retroposition of the ribosomal protein s23 mRNA and encodes proteins that decrease Alzheimer’s beta-amyloid level and tau phosphorylation. Hum Mol Genet. 2010;19:3835–43.
Zhao D, Zhou Y, Huo Y, Meng J, Xiao X, Han L, et al. RPS23RG1 modulates tau phosphorylation and axon outgrowth through regulating p35 proteasomal degradation. Cell Death Differ. 2021;28:337–48.
Yan L, Chen Y, Li W, Huang X, Badie H, Jian F, et al. RPS23RG1 reduces Abeta oligomer-induced synaptic and cognitive deficits. Sci Rep. 2016;6:18668.
Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–7.
Tang PM, Nikolic-Paterson DJ, Lan HY. Macrophages: versatile players in renal inflammation and fibrosis. Nat Rev Nephrol. 2019;15:144–58.
Tarasewicz E, Jeruss JS. Phospho-specific Smad3 signaling: impact on breast oncogenesis. Cell Cycle. 2012;11:2443–51.
Zelenitsky SA, Zhanel GG. Phenazopyridine in urinary tract infections. Ann Pharmacother. 1996;30:866–8.
Gaines KK. Phenazopyridine hydrochloride: the use and abuse of an old standby for UTI. Urol Nurs. 2004;24:207–9.
McClure R, Redha R, Vinson P, Pham W. A robust and scalable high-throughput compatible assay for screening amyloid-beta-binding compounds. J Alzheimers Dis. 2019;70:187–97.
Drummond E, Wisniewski T. Alzheimer’s disease: experimental models and reality. Acta Neuropathol. 2017;133:155–75.
Sasaguri H, Hashimoto S, Watamura N, Sato K, Takamura R, Nagata K, et al. Recent advances in the modeling of Alzheimer’s disease. Front Neurosci. 2022;16:807473.
Malm TM, Iivonen H, Goldsteins G, Keksa-Goldsteine V, Ahtoniemi T, Kanninen K, et al. Pyrrolidine dithiocarbamate activates Akt and improves spatial learning in APP/PS1 mice without affecting beta-amyloid burden. J Neurosci. 2007;27:3712–21.
Flanders KC, Lippa CF, Smith TW, Pollen DA, Sporn MB. Altered expression of transforming growth factor-beta in Alzheimer’s disease. Neurology. 1995;45:1561–9.
Ueberham U, Ueberham E, Gruschka H, Arendt T. Altered subcellular location of phosphorylated Smads in Alzheimer’s disease. Eur J Neurosci. 2006;24:2327–34.
Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, et al. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer’s pathology. J Clin Invest. 2006;116:3060–9.
Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, et al. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14:681–7.
Xu L, Pan CL, Wu XH, Song JJ, Meng P, Li L, et al. Inhibition of Smad3 in macrophages promotes Abeta efflux from the brain and thereby ameliorates Alzheimer’s pathology. Brain Behav Immun. 2021;95:154–67.
Zhang Y, Feng XH, Derynck R. Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-beta-induced transcription. Nature. 1998;394:909–13.
Terstappen GC, Meyer AH, Bell RD, Zhang W. Strategies for delivering therapeutics across the blood-brain barrier. Nat Rev Drug Disco. 2021;20:362–83.
Mansor NI, Nordin N, Mohamed F, Ling KH, Rosli R, Hassan Z. Crossing the blood-brain barrier: a review on drug delivery strategies for treatment of the central nervous system diseases. Curr Drug Deliv. 2019;16:698–711.
Funding
This work was supported by grants from National Natural Science Foundation of China (82130039 and U21A20361 to YwZ; 82001442 to DZ, and 92049202 and 92149303 to HX), National Key Research and Development Program of China (2018YFC2000400 to YwZ), and The Major Program of Brain Science and Brain-Like Intelligence Technology (2021ZD0202400 to HX).
Author information
Authors and Affiliations
Contributions
CW and YwZ designed research. CW and YZ carried out most molecular and animal studies. DZ, YH, and JX performed some molecular experiments. XZ and HL provided technical support. HX helped on data interpretation. YwZ supervised the project. CW and YwZ wrote the manuscript. All authors reviewed and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, C., Zhang, Y., Zhao, D. et al. Phenazopyridine promotes RPS23RG1/Rps23rg1 transcription and ameliorates Alzheimer-associated phenotypes in mice. Neuropsychopharmacol. 47, 2042–2050 (2022). https://doi.org/10.1038/s41386-022-01373-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-022-01373-7
This article is cited by
-
NAD + in fatty liver disease: mechanistic insights and associated targets
Cell Biology and Toxicology (2025)