Abstract
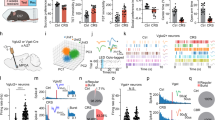
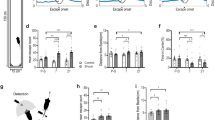
Effective coping plays an important role in preventing stress-induced neuropsychiatric conditions. The ventromedial prefrontal cortex (vmPFC) has been associated with active, adaptive coping in humans and rodents. Chronic or severe stress has been shown to induce a maladaptive shift from active to passive coping behavior; however, the neural circuits for effective coping strategies remain unclear. In the current study, we demonstrated that neurons in the infralimbic (IL) subregion of rat vmPFC that project to the lateral septum (LS) were recruited by exposure to the shock probe in the shock-probe defensive burying (SPDB) test. Both chemogenetic inhibition of LS-projecting neurons in the IL and optogenetic inhibition of glutamatergic IL terminals in the LS selectively suppressed active burying responses in the SPDB test in non-stressed rats. In contrast, chemogenetic activation of the IL-LS pathway effectively reversed the shift from active coping to passive immobility in the SPDB test induced by chronic unpredictable stress (CUS). These results indicate that top-down regulation of the LS by a projection from the IL cortex is necessary for an active, adaptive behavioral coping response, and is sufficient to restore active coping that has been compromised by chronic stress. More broadly, these results point to the IL-to-LS circuit as a potential substrate underlying maladaptive shifts from active to passive coping behavior that are often associated with stress-related neuropsychiatric disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Primary data generated, analyzed and included in the results reported in the current study are available from the corresponding author upon reasonable written request.
References
Kessler RC. The effects of stressful life events on depression. Annu Rev Psychol. 1997;48:191–214.
Maeng LY, Milad MR Post-Traumatic Stress Disorder: The Relationship Between the Fear Response and Chronic Stress. Chronic Stress (Thousand Oaks). 2017:eCollection
Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychol Med. 1997;27:1101–19.
LeDoux JE, Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry. 2001;158:1953–5.
Olff M, Langeland W, Gersons BP. Effects of appraisal and coping on the neuroendocrine response to extreme stress. Neurosci Biobehav Rev. 2005;29:457–67.
Charney DS. Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. Am J Psychiatry. 2004;161:195–216.
Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, et al. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–35.
Gutner CA, Rizvi SL, Monson CM, Resick PA. Changes in coping strategies, relationship to the perpetrator, and posttraumatic distress in female crime victims. J Trauma Stress. 2006;19:813–23.
Girotti M, Adler SM, Bulin SE, Fucich EA, Paredes D, Morilak DA. Prefrontal cortex executive processes affected by stress in health and disease. Prog Neuropsychopharmacol Biol Psychiatry. 2018;85:161–79.
Maier SF, Watkins LR. Role of the medial prefrontal cortex in coping and resilience. Brain Res. 2010;1355:52–60.
Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci USA. 2016;113:8837–42.
Bondi CO, Barrera G, Lapiz MD, Bedard T, Mahan A, Morilak DA. Noradrenergic facilitation of shock-probe defensive burying in lateral septum of rats, and modulation by chronic treatment with desipramine. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:482–95.
Johnson SB, Lingg RT, Skog TD, Hinz DC, Romig-Martin SA, Viau V, et al. Activity in a prefrontal-periaqueductal gray circuit overcomes behavioral and endocrine features of the passive coping stress response. Proc Natl Acad Sci USA. 2022;119:e2210783119.
Korte SM, Bouws GA, Koolhaas JM, Bohus B. Neuroendocrine and behavioral responses during conditioned active and passive behavior in the defensive burying/probe avoidance paradigm: effects of ipsapirone. Physiol Behav. 1992;52:355–61.
De Boer SF, Slangen JL, Van der Gugten J. Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: effects of chlordiazepoxide. Physiol Behav. 1990;47:1089–98.
Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA. Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology. 2015;232:3123–33.
Fucich EA, Paredes D, Saunders MO, Morilak DA. Activity in the ventral medial prefrontal cortex is necessary for the therapeutic effects of extinction in rats. J Neurosci. 2018;38:1408–17.
Fucich EA, Paredes D, Morilak DA. Therapeutic effects of extinction learning as a model of exposure therapy in rats. Neuropsychopharmacology. 2016;41:3092–102.
Hatherall L, Sanchez C, Morilak DA. Chronic vortioxetine treatment reduces exaggerated expression of conditioned fear memory and restores active coping behavior in chronically stressed rats. Int J Neuropsychopharmacol. 2017;20:316–23.
Fucich EA, Morilak DA. Shock-probe defensive burying test to measure active versus passive coping style in response to an aversive stimulus in rats. Bio Protoc. 2018;8:e2998.
Shah AA, Sjovold T, Treit D. Inactivation of the medial prefrontal cortex with the GABAA receptor agonist muscimol increases open-arm activity in the elevated plus-maze and attenuates shock-probe burying in rats. Brain Res. 2004;1028:112–5.
Shah AA, Treit D. Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 2003;969:183–94.
Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res Brain Res Rev. 2004;46:71–117.
Wirtshafter HS, Wilson MA. Lateral septum as a nexus for mood, motivation, and movement. Neurosci Biobehav Rev. 2021;126:544–59.
Patel H. The role of the lateral septum in neuropsychiatric disease. J Neurosci Res. 2022;100:1422–37.
Mongeau R, Miller GA, Chiang E, Anderson DJ. Neural correlates of competing fear behaviors evoked by an innately aversive stimulus. J Neurosci. 2003;23:3855–68.
Wang D, Pan X, Zhou Y, Wu Z, Ren K, Liu H, et al. Lateral septum-lateral hypothalamus circuit dysfunction in comorbid pain and anxiety. Mol Psychiatry. 2023;28:1090–100.
Pesold C, Treit D. Excitotoxic lesions of the septum produce anxiolytic effects in the elevated plus-maze and the shock-probe burying tests. Physiol Behav. 1992;52:37–47.
Degroot A, Kashluba S, Treit D. Septal GABAergic and hippocampal cholinergic systems modulate anxiety in the plus-maze and shock-probe tests. Pharm Biochem Behav. 2001;69:391–9.
Buchanan SL, Thompson RH, Maxwell BL, Powell DA. Efferent connections of the medial prefrontal cortex in the rabbit. Exp Brain Res. 1994;100:469–83.
Chiba T, Kayahara T, Nakano K. Efferent projections of infralimbic and prelimbic areas of the medial prefrontal cortex in the Japanese monkey, Macaca fuscata. Brain Res. 2001;888:83–101.
Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58.
Liu J, Tabisola K, Knippenberg AR, Ferreira LF, Morilak DA. The role of the projection from medial prefrontal cortex to lateral septum in coping behaviors. Neurosci Meet Planner Soc Neurosci 2023, online program no. 036.05.
Treit D, Pesold C. Septal lesions inhibit fear reactions in two animal models of anxiolytic drug action. Physiol Behav. 1990;47:365–71.
Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:913–23.
Bulin SE, Hohl KM, Paredes D, Silva JD, Morilak DA. Bidirectional optogenetically-induced plasticity of evoked responses in the rat medial prefrontal cortex can impair or enhance cognitive set-shifting. eNeuro. 2020;7:ENEURO.0363–19.2019.
Paredes D, Knippenberg AR, Morilak DA. Infralimbic BDNF signaling is necessary for the beneficial effects of extinction on set shifting in stressed rats. Neuropsychopharmacology. 2021;47:507–15.
Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 6th Edition. Academic Press: San Diego; 2007.
Cullinan WE, Herman JP, Battaglia DF, Akil H, Watson SJ. Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience. 1995;64:477–505.
Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008;36:129–39.
Hohne N, Poidinger M, Merz F, Pfister H, Bruckl T, Zimmermann P, et al. Increased HPA axis response to psychosocial stress in remitted depression: the influence of coping style. Biol Psychol. 2014;103:267–75.
Tiet QQ, Rosen C, Cavella S, Moos RH, Finney JW, Yesavage J. Coping, symptoms, and functioning outcomes of patients with posttraumatic stress disorder. J Trauma Stress. 2006;19:799–811.
Hassija CM, Luterek JA, Naragon-Gainey K, Moore SA, Simpson T. Impact of emotional approach coping and hope on PTSD and depression symptoms in a trauma exposed sample of Veterans receiving outpatient VA mental health care services. Anxiety Stress Coping. 2012;25:559–73.
Pinel JPT D. Burying as a defensive response in rats. J Comp Physiol Psychol 92: 708-712. J Comp Psychol. 1978;92:708–12.
De Boer SF, Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharm. 2003;463:145–61.
Treit D, Pesold C, Rotzinger S. Dissociating the anti-fear effects of septal and amygdaloid lesions using two pharmacologically validated models of rat anxiety. Behav Neurosci. 1993;107:770–85.
de Leon Reyes NS, Sierra Diaz P, Nogueira R, Ruiz-Pino A, Nomura Y, de Solis CA, et al. Corticotropin-releasing hormone signaling from prefrontal cortex to lateral septum suppresses interaction with familiar mice. Cell. 2023;186:4152–71.e31.
Carus-Cadavieco M, Gorbati M, Ye L, Bender F, van der Veldt S, Kosse C, et al. Gamma oscillations organize top-down signalling to hypothalamus and enable food seeking. Nature. 2017;542:232–36.
Chen YH, Wu JL, Hu NY, Zhuang JP, Li WP, Zhang SR, et al. Distinct projections from the infralimbic cortex exert opposing effects in modulating anxiety and fear. J Clin Invest. 2021;131:e145692.
Kirby LG, Lucki I. The effect of repeated exposure to forced swimming on extracellular levels of 5-hydroxytryptamine in the rat. Stress. 1998;2:251–63.
Thomas E, Pernar L, Lucki I, Valentino RJ. Corticotropin-releasing factor in the dorsal raphe nucleus regulates activity of lateral septal neurons. Brain Res. 2003;960:201–8.
Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–76.
VanRyzin JW, Marquardt AE, McCarthy MM. Developmental origins of sex differences in the neural circuitry of play. Int J Play. 2020;9:58–75.
Brady JV, Nauta WJ. Subcortical mechanisms in emotional behavior: affective changes following septal forebrain lesions in the albino rat. J Comp Physiol Psychol. 1953;46:339–46.
Albert DJ, Richmond SE. Hyperreactivity and aggressiveness following infusion of local anesthetic into the lateral septum or surrounding structures. Behav Biol. 1976;18:211–26.
Rizzi-Wise CA, Wang DV. Putting together pieces of the lateral septum: multifaceted functions and its neural pathways. eNeuro. 2021;8:ENEURO.0315–21.2021.
Johnson SB, Emmons EB, Lingg RT, Anderson RM, Romig-Martin SA, LaLumiere RT, et al. Prefrontal-bed nucleus circuit modulation of a passive coping response set. J Neurosci. 2019;39:1405–19.
Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–38.
Rafnsson FD, Jonsson FH, Windle M. Coping strategies, stressful life events, problem behaviors, and depressed affect. Anxiety, Stress Coping. 2006;19:241–57.
Johnsen BH, Eid J, Laberg JC, Thayer JF. The effect of sensitization and coping style on post-traumatic stress symptoms and quality of life: two longitudinal studies. Scand J Psychol. 2002;43:181–88.
Olff M, Langeland W, Gersons BP. The psychobiology of PTSD: coping with trauma. Psychoneuroendocrinology. 2005;30:974–82.
Korem N, Ben-Zion Z, Spiller TR, Duek OA, Harpaz-Rotem I, Pietrzak RH. Correlates of avoidance coping in trauma-exposed U.S. military veterans: Results from the National Health and Resilience in Veterans Study. J Affect Disord. 2023;339:89–97.
Willner P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress. 2017;6:78–93.
Khan AR, Geiger L, Wiborg O, Czeh B. Stress-induced morphological, cellular and molecular changes in the brain-lessons learned from the chronic mild stress model of depression. Cells. 2020;9:1026.
Serran GA, Firestone P, Marshall WL, Moulden H. Changes in coping following treatment for child molesters. J Interpers Violence. 2007;22:1199–210.
Ihara S, Katayama N, Nogami W, Amano M, Noda S, Kurata C, et al. Comparison of changes in stress coping strategies between cognitive behavioral therapy and pharmacotherapy. Front Psychiatry. 2024;15:1343637.
Boden MT, Bonn-Miller MO, Vujanovic AA, Drescher KD. A Prospective Investigation of Changes in Avoidant and Active Coping and Posttraumatic Stress Disorder Symptoms among Military Veteran. J Psychopathol Behav Assess. 2012;34:433–39.
Acknowledgements
We thank Anna R. Knippenberg and Livia Ferreira for technical assistance. Confocal images were generated in the Core Optical Imaging Facility which is supported by UT Health San Antonio and NIH-NCI grant P30 CA54174.
Funding
This work was supported by research grant R01MH053851 from the National Institute of Mental Health, National Institutes of Health; by Merit Award I01BX003512 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Program; and by a grant from the William and Ella Owens Medical Research Foundation, none of which had any role in study design, data collection, analysis or interpretation, nor in the preparation or decision to submit this paper for publication. The contents of this paper do not represent the views of the Department of Veterans Affairs or the U.S. Government.
Author information
Authors and Affiliations
Contributions
JL contributed to experimental design and conception, data collection, analysis and interpretation of the results, and wrote and edited the manuscript. KT contributed to data collection and edited the manuscript. DAM was responsible for funding and resources necessary to conduct the research, contributed to experimental design and conception, data interpretation, editing and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Tabisola, K.M. & Morilak, D.A. A projection from the medial prefrontal cortex to the lateral septum modulates coping behavior on the shock-probe test. Neuropsychopharmacol. 50, 1245–1255 (2025). https://doi.org/10.1038/s41386-025-02074-7
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41386-025-02074-7
This article is cited by
-
Antidepressant-like effects of extinction learning as an animal model of behavioral therapy
Molecular Psychiatry (2025)