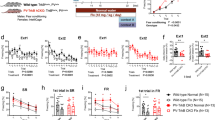
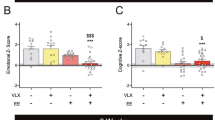
Abstract
Persistent passive coping (p-coping) behaviour is a hallmark feature in major depression and is reversed by fast-acting antidepressants (such as ketamine). This behaviour is regulated by a specific cortico-midbrain circuit. However, the contribution of inhibition in prefrontal cortex to p-coping modulation, and its relevance to chronic stress and/or fast-acting antidepressant effects, are poorly understood. Here, we found that rostral prelimbic cortex (rPL) bidirectionally controls p-coping behaviour where excitatory and inhibitory neurons play opposite roles. Chronic stress leads to a reduced excitation/inhibition (E/I) ratio, reflected as alterations of in vivo spiking rate, synaptic strength, and intrinsic excitability of rPL neurons. A fast-acting antidepressant, (2 R,6 R)-hydroxynorketamine (HNK), reduced p-coping, restored rPL E/I ratio, and partially reversed neuronal changes in chronically stressed mice. Notably, chronic stress and HNK significantly affected fast-spiking/parvalbumin inhibitory neurons which also bidirectionally regulate the passive coping behaviour, highlighting the critical roles of these neurons in the above processes. These findings underscore the importance of rPL E/I balance in regulating p-coping behaviour, which is disrupted by chronic stress and rapidly restored by fast-acting antidepressant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 13 print issues and online access
$259.00 per year
only $19.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or supplementary materials upon request.
References
Joëls M, Karst H, Sarabdjitsingh RA. The stressed brain of humans and rodents. Acta Physiol (Oxf). 2018;223:e13066.
de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75.
Herman JP. Neural control of chronic stress adaptation. Front Behav Neurosci. 2013;7:61.
Radley JJ, Herman JP. Preclinical models of chronic stress: adaptation or pathology? Biol Psychiatry. 2023;94:194–202.
Carroll L Passive Coping Strategies. In: Gellman MD, Turner JR, editors. Encyclopedia of behavioral medicine. New York, NY: Springer New York; 2013. p. 1442-.
Molendijk ML, de Kloet ER. Coping with the forced swim stressor: current state-of-the-art. Behav Brain Res. 2019;364:1–10.
Molendijk ML, de Kloet ER. Forced swim stressor: Trends in usage and mechanistic consideration. Eur J Neurosci. 2022;55:2813–31.
Johnson SB, Emmons EB, Lingg RT, Anderson RM, Romig-Martin SA, LaLumiere RT, et al. Prefrontal-Bed Nucleus Circuit Modulation of a Passive Coping Response Set. J Neurosci. 2019;39:1405–19.
Johnson SB, Lingg RT, Skog TD, Hinz DC, Romig-Martin SA, Viau V, et al. Activity in a prefrontal-periaqueductal gray circuit overcomes behavioral and endocrine features of the passive coping stress response. Proc Natl Acad Sci USA. 2022;119:e2210783119.
McKlveen JM, Moloney RD, Scheimann JR, Myers B, Herman JP. Braking” the prefrontal cortex: the role of glucocorticoids and interneurons in stress adaptation and pathology. Biol Psychiatry. 2019;86:669–81.
Cerniauskas I, Winterer J, de Jong JW, Lukacsovich D, Yang H, Khan F, et al. Chronic stress induces activity, synaptic, and transcriptional remodeling of the lateral habenula associated with deficits in motivated behaviors. Neuron. 2019;104:899–915.e8.
McKlveen JM, Morano RL, Fitzgerald M, Zoubovsky S, Cassella SN, Scheimann JR, et al. Chronic stress increases prefrontal inhibition: a mechanism for stress-induced prefrontal dysfunction. Biol Psychiatry. 2016;80:754–64.
Nawreen N, Cotella EM, Morano R, Mahbod P, Dalal KS, Fitzgerald M, et al. Chemogenetic inhibition of infralimbic prefrontal cortex GABAergic parvalbumin interneurons attenuates the impact of chronic stress in male mice. eNeuro. 2020;7:ENEURO.0423-19.
Grunebaum MF, Galfalvy HC, Choo TH, Keilp JG, Moitra VK, Parris MS, et al. Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry. 2018;175:327–35.
Phillips JL, Norris S, Talbot J, Birmingham M, Hatchard T, Ortiz A, et al. Single, repeated, and maintenance ketamine infusions for treatment-resistant depression: a randomized controlled trial. Am J Psychiatry. 2019;176:401–9.
Fogaça MV, Wu M, Li C, Li XY, Picciotto MR, Duman RS. Inhibition of GABA interneurons in the mPFC is sufficient and necessary for rapid antidepressant responses. Mol Psychiatry. 2021;26:3277–91.
Chen JY, Wu K, Guo MM, Song W, Huang ST, Zhang YM. The PrL(Glu)→avBNST(GABA) circuit rapidly modulates depression-like behaviors in male mice. iScience. 2023;26:107878.
Johnston JN, Kadriu B, Kraus C, Henter ID, Zarate CA Jr. Ketamine in neuropsychiatric disorders: an update. Neuropsychopharmacology. 2024;49:23–40.
Hess EM, Riggs LM, Michaelides M, Gould TD. Mechanisms of ketamine and its metabolites as antidepressants. Biochem Pharm. 2022;197:114892.
Riggs LM, Gould TD. Ketamine and the future of rapid-acting antidepressants. Annu Rev Clin Psychol. 2021;17:207–31.
Highland JN, Zanos P, Riggs LM, Georgiou P, Clark SM, Morris PJ, et al. Hydroxynorketamines: pharmacology and potential therapeutic applications. Pharm Rev. 2021;73:763–91.
Raja SM, Guptill JT, Mack M, Peterson M, Byard S, Twieg R, et al. A phase 1 assessment of the safety, tolerability, pharmacokinetics and pharmacodynamics of (2R,6R)-hydroxynorketamine in healthy volunteers. Clin Pharmacol Ther. 2024;116:1314–24.
Srikumar BN, Paschapur M, Kalidindi N, Adepu B, Das ML, Sreedhara MV, et al. Characterization of the adrenocorticotrophic hormone - induced mouse model of resistance to antidepressant drug treatment. Pharm Biochem Behav. 2017;161:53–61.
Walker AJ, Burnett SA, Hasebe K, McGillivray JA, Gray LJ, McGee SL, et al. Chronic adrenocorticotrophic hormone treatment alters tricyclic antidepressant efficacy and prefrontal monoamine tissue levels. Behav Brain Res. 2013;242:76–83.
Ma X, Yang S, Zhang Z, Liu L, Shi W, Yang S, et al. Rapid and sustained restoration of astrocytic functions by ketamine in depression model mice. Biochem Biophys Res Commun. 2022;616:89–94.
Kupferschmidt DA, Cummings KA, Joffe ME, MacAskill A, Malik R, Sánchez-Bellot C, et al. Prefrontal interneurons: populations, pathways, and plasticity supporting typical and disordered cognition in rodent models. J Neurosci. 2022;42:8468–76.
Wang T, Yan R, Zhang X, Wang Z, Duan H, Wang Z, et al. Paraventricular thalamus dynamically modulates aversive memory via tuning prefrontal inhibitory circuitry. J Neurosci. 2023;43:3630–46.
Bartolini G, Ciceri G, Marín O. Integration of GABAergic interneurons into cortical cell assemblies: lessons from embryos and adults. Neuron. 2013;79:849–64.
Meyer HC, Sangha S, Radley JJ, LaLumiere RT, Baratta MV. Environmental certainty influences the neural systems regulating responses to threat and stress. Neurosci Biobehav Rev. 2021;131:1037–55.
Yoon SH, Chung G, Song WS, Oh SP, Kim SJ, Kim M-H. Shifting hippocampal excitation/inhibition balance modifies despair-like behavior in mice. bioRxiv. 2020. 2020.02.18.953786.
Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, et al. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205.
Yildiz-Yesiloglu A, Ankerst DP. Review of 1H magnetic resonance spectroscopy findings in major depressive disorder: a meta-analysis. Psychiatry Res. 2006;147:1–25.
Arnone D, Mumuni AN, Jauhar S, Condon B, Cavanagh J. Indirect evidence of selective glial involvement in glutamate-based mechanisms of mood regulation in depression: meta-analysis of absolute prefrontal neuro-metabolic concentrations. Eur Neuropsychopharmacol. 2015;25:1109–17.
Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2019;24:952–64.
Ali F, Gerhard DM, Sweasy K, Pothula S, Pittenger C, Duman RS, et al. Ketamine disinhibits dendrites and enhances calcium signals in prefrontal dendritic spines. Nat Commun. 2020;11:72.
Fogaça MV, Daher F, Picciotto MR. Effects of ketamine on GABAergic and glutamatergic activity in the mPFC: biphasic recruitment of GABA function in antidepressant-like responses. Neuropsychopharmacology. 2025;50:673–84.
Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, et al. Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci USA. 2019;116:5160–9.
Duman RS, Shinohara R, Fogaça MV, Hare B. Neurobiology of rapid-acting antidepressants: convergent effects on GluA1-synaptic function. Mol Psychiatry. 2019;24:1816–32.
Gerhard DM, Pothula S, Liu RJ, Wu M, Li XY, Girgenti MJ, et al. GABA interneurons are the cellular trigger for ketamine’s rapid antidepressant actions. J Clin Invest. 2020;130:1336–49.
Ghosal S, Duman CH, Liu RJ, Wu M, Terwilliger R, Girgenti MJ, et al. Ketamine rapidly reverses stress-induced impairments in GABAergic transmission in the prefrontal cortex in male rodents. Neurobiol Dis. 2020;134:104669.
Li Y, Du Y, Wang C, Lu G, Sun H, Kong Y, et al. (2R,6R)-hydroxynorketamine acts through GluA1-induced synaptic plasticity to alleviate PTSD-like effects in rat models. Neurobiol Stress. 2022;21:100503.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6.
Wamsley B, Fishell G. Genetic and activity-dependent mechanisms underlying interneuron diversity. Nat Rev Neurosci. 2017;18:299–309.
Page CE, Coutellier L. Prefrontal excitatory/inhibitory balance in stress and emotional disorders: Evidence for over-inhibition. Neurosci Biobehav Rev. 2019;105:39–51.
Wohleb ES, Wu M, Gerhard DM, Taylor SR, Picciotto MR, Alreja M, et al. GABA interneurons mediate the rapid antidepressant-like effects of scopolamine. J Clin Invest. 2016;126:2482–94.
Malik R, Li Y, Schamiloglu S, Sohal VS. Top-down control of hippocampal signal-to-noise by prefrontal long-range inhibition. Cell. 2022;185:1602–17.e17.
Wang Q, Jie W, Liu JH, Yang JM, Gao TM. An astroglial basis of major depressive disorder? An overview. Glia. 2017;65:1227–50.
Lu CL, Ren J, Cao X. An astroglial basis of major depressive disorder: molecular, cellular, and circuit features. Biol Psychiatry. 2024;97:217–26.
Murphy-Royal C, Gordon GR, Bains JS. Stress-induced structural and functional modifications of astrocytes-Further implicating glia in the central response to stress. Glia. 2019;67:1806–20.
Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14:1225–36.
Sanacora G, Banasr M. From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry. 2013;73:1172–9.
Rouach N, Koulakoff A, Abudara V, Willecke K, Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–5.
Østergaard L, Jørgensen MB, Knudsen GM. Low on energy? An energy supply-demand perspective on stress and depression. Neurosci Biobehav Rev. 2018;94:248–70.
Jiang T, Feng M, Hutsell A, Lüscher B. Sex-specific GABAergic microcircuits that switch vulnerability into resilience to stress and reverse the effects of chronic stress exposure. Mol Psychiatry. 2025;30:2297–308.
Shepard R, Page CE, Coutellier L. Sensitivity of the prefrontal GABAergic system to chronic stress in male and female mice: Relevance for sex differences in stress-related disorders. Neuroscience. 2016;332:1–12.
Li S, Zhang X, Cai Y, Zheng L, Pang H, Lou L. Sex difference in incidence of major depressive disorder: an analysis from the Global Burden of Disease Study 2019. Ann Gen Psychiatry. 2023;22:53.
Hu Y, Zhang X, Fong TH, Wang T, Zhang J, Zhang Y, et al. Distinct subpopulations of parvalbumin neurons participating in divergent prefrontal functions. Neuropsychopharmacology. 2025;50:1502–14.
Fukumoto K, Fogaça MV, Liu RJ, Duman C, Kato T, Li XY, et al. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci USA. 2019;116:297–302.
Riggs LM, Aracava Y, Zanos P, Fischell J, Albuquerque EX, Pereira EFR, et al. (2R,6R)-hydroxynorketamine rapidly potentiates hippocampal glutamatergic transmission through a synapse-specific presynaptic mechanism. Neuropsychopharmacology. 2020;45:426–36.
Yao N, Skiteva O, Zhang X, Svenningsson P, Chergui K. Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Mol Psychiatry. 2018;23:2066–77.
Chen Y, Yan P, Wei S, Zhu Y, Lai J, Zhou Q. Ketamine metabolite alleviates morphine withdrawal-induced anxiety via modulating nucleus accumbens parvalbumin neurons in male mice. Neurobiol Dis. 2023;186:106279.
Acknowledgements
We thank the members of Zhou’s lab for the technical supports and helpful discussion. This work is supported by grant from Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions (2023SHIBS0004), National Natural Science Foundation of China (82471548), and Wenzhou Basic Scientific Research Project (Y2023064).
Author information
Authors and Affiliations
Contributions
Conceptualization: QZ, THF, and XM. Methodology: QZ, THF, TL, XM, and XC. Experiment and data analysis: THF, TL, and QZ. Writing—original draft: QZ and THF. Writing—review and editing: QZ, THF, and XC. Funding acquisition: QZ and XC. Project administration: QZ. Supervision, QZ.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fong, T.H., Li, T., Ma, X. et al. Prefrontal contribution to passive coping behaviour in chronic stress and treatment by fast-acting antidepressant. Neuropsychopharmacol. (2025). https://doi.org/10.1038/s41386-025-02200-5
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41386-025-02200-5