Abstract
Family and twin studies indicate that 20–60% of the vulnerability to opioid use disorder (OUD) is influenced by genetic factors, but the specific genes driving addiction-like behaviors, including sensitivity to opioid analgesia, tolerance, dependence, and escalation of oxycodone self-administration, remain unidentified, limiting precision medicine approaches. To address this, we phenotyped over 500 heterogeneous stock (HS) rats, an outbred population with high genetic diversity, to characterize traits associated with OUD vulnerability and resilience. Rats self-administered oxycodone (150 µg/kg/infusion) in short-access (2 h/day, 4 days) followed by long-access (12 h/day, 14 days) sessions. We assessed motivation for oxycodone using progressive ratio testing, withdrawal-induced hyperalgesia with von Frey tests, and tolerance to oxycodone’s analgesic effects via tail immersion tests. Large cohorts (n = 46–60) and Z-score normalization minimized cohort-specific effects. An Addiction Index, derived from averaging Z-scores of escalation, motivation, tolerance, and hyperalgesia, revealed significant individual variability. Rats with severe addiction-like behaviors displayed higher initial analgesia, greater escalation, and more pronounced tolerance compared to resilient rats. Females showed increased escalation and motivation compared to males, but similar tolerance and hyperalgesia. Principal component analysis confirmed the Addiction Index’s validity, accounting for 40% of behavioral variance. This high-throughput phenotyping in HS rats, leveraging their genetic diversity, provides a robust framework for genome-wide association studies to identify gene variants linked to OUD vulnerability, offering translational potential for discovering novel therapeutic targets and advancing pharmacogenetic strategies for OUD treatment.
Similar content being viewed by others
Introduction
The opioid crisis remains a pressing public health challenge, with over 2 million Americans affected by opioid use disorder [1]. Over the past 15 years, oxycodone consumption has surged by ~500%, paralleled by a quadrupling of opioid-related overdose deaths [2, 3]. While opioids effectively manage acute and chronic pain for some patients [4], their addiction risk complicates clinical decision-making, as little is known about how pain mechanisms interact with prescription opioids to drive addiction vulnerability.
Twin studies have estimated the heritability of OUD from 20 to 60% [5,6,7]. Human genome-wide association studies (GWAS) have identified loci associated with addiction-related traits [8,9,10,11,12,13,14], and more recent work has specifically examined OUD and related traits [15,16,17,18,19,20]. Despite this progress, the genetic and biological mechanisms mediating individual differences in opioid effects and addiction-like behaviors are poorly defined [21, 22].
Advances in preclinical GWAS methodologies have overcome previous limitations, such as insufficient recombination, by using outbred populations like heterogeneous stock (HS) rats, which are a genetically diverse outbred population created by interbreeding eight inbred strains that have undergone extensive recombination to enable fine-scale genetic mapping [23, 24]. Preclinical models offer standardized, longitudinal, and quantitative behavioral assessments in controlled environments, alongside opportunities for genetic manipulation.
A key advancement in preclinical opioid research is the extended-access oxycodone self-administration model, which exhibits high face, predictive, and construct validity for OUD [22, 25,26,27]. Unlike human studies, where GWAS for substance-specific OUD are lacking, preclinical models can identify novel genetic targets for specific substances, such as heroin [28,29,30] or oxycodone. Here, we used the extended-access oxycodone self-administration model to phenotype >500 HS rats. Rats were assessed for escalation, motivation, analgesia, tolerance, and withdrawal-induced hyperalgesia, integrated into an Addiction Index [31] to distinguish vulnerable and resilient individuals. This approach lays a robust foundation for GWAS to uncover genetic variants driving oxycodone addiction-like behaviors.
Methods
Detailed procedures can be found in the George lab protocol repository on protocols.io (https://www.protocols.io/view/oxycodone-iv-self-administration-14egnze2pg5d/v1).
Animals
HS rats (Rat Genome Database NMcwiWFsm #13673907, n = 542), developed at the NIH in the 1980s to maximize genetic diversity [32], were provided by Dr. Leah Solberg Woods (Medical College of Wisconsin, now at Wake Forest University School of Medicine). HS rats were derived from interbreeding eight inbred rat strains (ACI/N, BN/SsN, BUF/N, F344/N, M520/N, MR/N, WKY/N, and WN/N) and maintained with a large number of breeder pairs using a randomized breeding strategy, with each pair contributing one male and one female per generation to minimize inbreeding and genetic drift [24]. Each rat was implanted with an RFID chip. Rats were shipped at 3–4 weeks of age, quarantined for 2 weeks, and housed in pairs in a temperature- (20–22 °C) and humidity-controlled (45–55%) vivarium with a 12 h/12 h reversed light/dark cycle. They had ad libitum access to tap water and food pellets (PJ Noyes Company, Lancaster, NH, USA). A total of 700 HS rats were initially used in the intravenous oxycodone self-administration study. Complete and usable data were ultimately obtained from 542 animals, corresponding to an attrition rate of 22.6% (158 rats). Animals were excluded for one or more of the following reasons: loss of catheter patency or inconsistent drug delivery, post-surgical complications (including implantation failures, infections or sepsis requiring euthanasia on animal-welfare grounds), hardware or infusion-system malfunctions, severe opioid-induced respiratory depression (overdose), general health deterioration (≥20% body-weight loss, marked lethargy, or other signs of distress necessitating euthanasia), missing, corrupted, or incomplete experimental records. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of The Scripps Research Institute and University of California, San Diego.
Drugs
Oxycodone hydrochloride (National Institute on Drug Abuse, Bethesda, MD, USA) was dissolved in 0.9% sterile saline and administered intravenously at 150 μg/kg/infusion (free base, 0.1 ml volume over 6 s). This dose was selected based on previous studies demonstrating reliable self-administration and escalation in rats [31, 33, 34], as well as its ability to achieve plasma concentrations (~40 ng/ml) associated with pharmacological effects [35]. To ensure that all rats received the same dose of oxycodone in mg/kg despite the fixed infusion duration, the concentration of oxycodone was individually adjusted in each syringe according to the animal’s body weight.
Intravenous catheterization
Rats were anesthetized with vaporized isoflurane (1–5%) and implanted with intravenous catheters in the right jugular vein using established procedures described previously [31]. Catheters consisted of 18 cm Micro-Renathane tubing (0.023-inch inner diameter, 0.037-inch outer diameter; Braintree Scientific, Braintree, MA, USA) connected to a 90°-angled guide cannula (Plastics One, Roanoke, VA, USA), secured in dental acrylic and anchored with a 2 cm diameter, 1 mm thick mesh. Tubing was inserted into the vein following a needle puncture (22 G) and secured with a suture. The guide cannula was punctured through a small incision on the back. The cannula exited through a small dorsal incision and was sealed with a plastic cap and metal cover to ensure sterility and protection. Post-surgery, rats received flunixin (2.5 mg/kg, s.c.) for analgesia and cefazolin (330 mg/kg, i.m.) for infection prophylaxis. Catheters were flushed daily with heparinized saline (10 U/ml of heparin sodium; American Pharmaceutical Partners, Schaumberg, IL, USA) in 0.9% bacteriostatic sodium chloride (Hospira, Lake Forest, IL, USA) containing cefazolin (52.4 mg/0.2 ml). Rats recovered for 7 days before self-administration, with daily monitoring to ensure catheter patency and animal welfare.
Behavioral testing
Operant self-administration
Oxycodone self-administration (SA) was conducted in operant conditioning chambers (29 × 24 × 19.5 cm; Med Associates, St. Albans, VT, USA) housed within sound-attenuating, ventilated cubicles. Chambers were constructed of transparent plastic front and back walls, opaque metal side walls, two retractable levers, and a cue light on the front panel. Sessions began with lever extension. Responses on the right (active) lever delivered oxycodone (150 μg/kg/infusion in 0.9% saline, 0.1 ml over 6 s) via an infusion pump connected to intravenous catheters, following a fixed-ratio 1 schedule. Each infusion was followed by a 20-s timeout, signaled by a cue light above the active lever, during which additional presses were recorded but had no effect. Responses on the left (inactive) lever were recorded without consequences. Fluid delivery and behavioral data were controlled using MED-PC IV software (Med Associates). Rats were initially trained in four short-access (ShA) sessions (2 h/day) to establish stable self-administration. Subsequently, they underwent 14 long-access (LgA) sessions (12 h/day, Monday–Friday) to assess escalation of oxycodone intake, starting at the onset of the dark phase of the 12 h/12 h light/dark cycle. Food and water were available ad libitum during LgA sessions, and a fresh wooden block (3 × 3 × 3 cm) was provided daily to enrich the self-administration chambers.
Progressive ratio testing
Rats were tested on a progressive ratio (PR) schedule of reinforcement at the end of each phase (once after ShA and once after LgA, see Fig. 1 for a detailed timeline). In the PR paradigm, the number of lever presses required for each oxycodone infusion (150 µg/kg/infusion) increased progressively within each session as follows: 1, 1, 2, 2, 3, 3, 4, 4, 6, 6, 8, 8, 10, 11, 12, 13, 14, 15, 16, 17, etc +1 until 50, 60, 70, 80, 90, 100. The breakpoint was defined as the last completed ratio before a 60-min period without completing the next ratio, terminating the session [36].
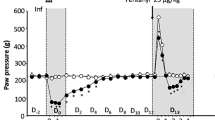
A Timeline of the behavioral paradigms. B Number of oxycodone infusions (150 μg/kg/infusion) during the first hour of self-administration under ShA (2 h/day), and LgA (12 h/day) conditions across 14 cohorts (N = 542). Data show significant escalation of intake from day 4 of LgA onward (***p < 0.001 vs. first LgA session, one-way ANOVA with Bonferroni post hoc comparisons). C Violin plot depicting the number of oxycodone infusions during PR testing after ShA and LgA phases (N = 542, ***p < 0.001, paired t-test). D Development of tolerance to oxycodone’s analgesic effects. Tail withdrawal thresholds (in seconds) measured via the tail immersion test at baseline (BSL), after three oxycodone infusions pre-extended-access self-administration (Oxy-pre-SA), and post-extended-access self-administration (Oxy post-SA) (***p < 0.001 vs BSL and ###p < 0.001 vs Oxy-pre-SA, one-way ANOVA with Bonferroni post hoc comparisons). E Development of withdrawal-induced hyperalgesia. Data represent percentage change from baseline in paw withdrawal force (g) measured via the von Frey test before and after extended-access oxycodone self-administration (***p < 0.001 vs BSL, paired t-test).
Mechanical nociceptive von Frey testing
Withdrawal-induced hyperalgesia was assessed using a dynamic plantar aesthesiometer (Ugo Basile, Gemonio, Italy) adapted from established protocols [37,38,39]. Mechanical sensitivity was measured by applying a linearly increasing force to the plantar surface of each hind paw until withdrawal occurred. The force (in grams) and latency to paw withdrawal were recorded, with three measurements per paw averaged for each rat. Testing was conducted at two time points: (i) baseline (prior to oxycodone exposure) and (ii) during acute withdrawal, 10–12 h after the final long-access (LgA) oxycodone self-administration session (see Fig. 1A for timeline). Baseline data are expressed as force (g), while withdrawal data are reported as the percent change in force from each rat’s baseline to enable within-subject evaluation of hyperalgesia.
Analgesia by tail immersion testing
Rats were gently restrained in a soft cloth pocket, and the distal half of the tail was submerged in a 52 °C water bath. Latency to tail withdrawal was recorded, with a 10 s cut-off to prevent tissue damage. Two measurements, taken 30 s apart, were averaged for each rat. Testing occurred at three time points: (i) baseline (pre-drug exposure), (ii) prior to oxycodone ShA self-administration, 15 min after two intravenous infusions of oxycodone (150 μg/kg, 0.1 ml volume), and (iii) 2 h post the final long-access (LgA) session (day 14), 15 min after two identical oxycodone infusions. The post-LgA test evaluated tolerance to oxycodone’s analgesic effects. The 15 min post-infusion timing was chosen based on peak plasma oxycodone concentrations and the 12 h withdrawal period aligned with prior studies assessing opioid tolerance [40].
Statistical analyses
All data were analyzed using Prism 9.0 (GraphPad, San Diego, CA, USA). Self-administration data, including escalation of oxycodone intake during short-access (ShA; 2 h/day) and long-access (LgA; 12 h/day) sessions, were analyzed using repeated-measures one-way analysis of variance (ANOVA) with session as the within-subject factor, followed by Bonferroni post hoc tests to compare oxycodone infusions across days. For sex differences in self-administration, a two-way repeated-measures ANOVA was used with sex as the between-subject factor and session as the within-subject factor, followed by Bonferroni post hoc tests. Progressive ratio (PR) data were analyzed using a two-way ANOVA with sex or group (resilient, mild, moderate, severe) as the between-subject factor and time (post-ShA vs. post-LgA) as the within-subject factor, followed by Bonferroni post hoc tests for pairwise comparisons. Tail immersion test data, assessing oxycodone’s analgesic effects and tolerance, were analyzed using a two-way ANOVA with group or sex as the between-subject factor and time (baseline, pre-ShA, post-LgA) as the within-subject factor, followed by Bonferroni post hoc tests. Von Frey test data, evaluating withdrawal-induced hyperalgesia, were analyzed using paired t-tests to compare baseline and post-LgA withdrawal thresholds within groups, and one-way ANOVA with Bonferroni post hoc tests for between-group comparisons. Pairwise comparisons between groups (e.g., resilient vs. severe) were conducted using unpaired Student’s t-tests. Pearson’s correlation analysis was used to examine relationships between behavioral measures (e.g., escalation, motivation, tolerance, hyperalgesia). To account for cohort variability, behavioral data from 14 cohorts (n = 46–60 per cohort) were normalized using Z-scores, calculated as Z = (x−μ)/σ, where x is the raw value, μ is the cohort mean, and σ is the cohort standard deviation. The Addiction Index was computed as the mean of Z-scores for escalation, PR breakpoint, tolerance, and hyperalgesia. Principal component analysis (PCA) was performed to validate the Addiction Index, retaining components with eigenvalues > 1, using R (version 4.2.1). Data are reported as mean ± SEM unless otherwise specified, with statistical significance set at p < 0.05. All tests were two-tailed, and exact p-values are provided where possible. More analyses are provided in the Supplementary Material (Figs. S1–S3).
Results
Evaluation of oxycodone addiction-like behaviors in HS rats
Addiction-like behaviors were assessed in 542 HS rats across 14 cohorts (n = 46–60 per cohort) using a standardized oxycodone self-administration protocol (Fig. 1A). Following jugular catheterization and a 7-day recovery, rats underwent four daily short-access (ShA; 2 h/day) sessions to establish oxycodone self-administration, followed by 14 daily long-access (LgA; 12 h/day) sessions to evaluate intake escalation. During the extended-access oxycodone self-administration phase, animals showed significant escalation of intake, as measured by the significant increase in oxycodone rewards per hour from day 4 of LgA onward compared to the first day of LgA (Fig. 1B): F(17,8687) = 205.2, p < 0.0001, one-way ANOVA, with Bonferroni post hoc comparisons. Inactive lever responding remained very low (<15 responses/session in most animals) showing negligible inter-individual variability (Fig. S1). Motivation for oxycodone was assessed after both ShA and LgA phases using a progressive ratio (PR) test (Fig. 1C). The number of oxycodone infusions significantly increased after extended-access of oxycodone self-administration, compared to levels at the end of the short-access phase (t541 = 18.25, p < 0.001). Given that PR data exhibit a non-normal distribution characterized by a significant floor effect and right skew, these results are also presented on a logarithmic scale in Fig. S2 to visualize the distribution of lower-responding subjects. Rats were also tested for baseline pain response, oxycodone’s analgesic effects, and the development of tolerance to these effects using the tail immersion test at three different phases of the behavioral protocol (see Fig. 1A for a detailed testing timeline). As shown in Fig. 1D, HS rats developed tolerance to the analgesic effects of oxycodone following extended-access to oxycodone self-administration (F(2,1076) = 279.4, p < 0.0001, one-way ANOVA). Bonferroni post hoc comparisons revealed a significant decline in oxycodone’s analgesic effects after 14 days of extended-access of oxycodone self-administration (p < 0.001, Fig. 1D). Finally, oxycodone withdrawal-induced hyperalgesia was assessed using the von Frey Test. Paw withdrawal responses to a mechanical stimulus were measured at the beginning and at the end of the behavioral paradigm (pre- and post- oxycodone, Fig. 1E), with data expressed as a percentage reduction in force (g) compared to baseline responses. Results demonstrated that HS rats developed withdrawal-induced hyperalgesia following extended-access oxycodone self-administration (t541 = 7.41, p < 0.001, Fig. 1E).
Sex differences in oxycodone addiction-like behaviors in HS rats
No sex differences were observed during the acquisition phase under ShA (2 h/day) conditions. However, during LgA (12 h/day), female HS rats exhibited greater escalation of oxycodone intake compared to males (Fig. 2A). The two-way ANOVA with sex as the between-subjects factor and sessions as the within-subject factor revealed significant main effects of sex (F(1542) = 6.577; p < 0.001), sessions (F(17,9210) = 224.5; p < 0.001) and their interaction (F(17,9210) = 5.661; p < 0.001). However, calculation of the effect size for the main effect of sex yielded a partial eta squared (η2p) of 0.012, indicating that while statistically significant, biological sex accounted for a small proportion (~1.2%) of the total variance in intake. Bonferroni post hoc test demonstrated that females self-administered significantly more oxycodone than males from day 6 of the LgA phase (day 10 in Fig. 2A) through the end of the behavioral protocol (p < 0.01). In the progressive ratio (PR) test, a two-way ANOVA with sex as the between-subject factor and time (ShA vs. LgA) as the within-subject factor showed a significant main effect of sex (F(1540) = 8.28; p < 0.01), time (F(1540) = 337.2; p < 0.001), and their interaction (F(1,540) = 4.83; p < 0.05). Like intake, the effect size for sex on motivation was small (η2p = 0.015), suggesting that sex differences explain approximately 1.5% of the phenotypic variance. Bonferroni post hoc tests confirmed that motivation for oxycodone increased in both sexes from ShA to LgA (p < 0.001), with females displaying significantly higher motivation than males after LgA (p < 0.01, Figs. 2B and 5B). Male and female HS rats showed comparable baseline response in the tail immersion test, similar responses to oxycodone’s analgesic effects, and equivalent development of tolerance to these effects, with no significant treatment x sex interaction (F(2,1074) = 0.19; p = NS, Fig. 2C). Similarly, no sex differences were observed in the development of withdrawal-induced hyperalgesia, as assessed by paw withdrawal thresholds, in the von Frey test during withdrawal (t540 = 1.32; p = NS, Fig. 2D).
A Number of oxycodone infusions (150 μg/kg/infusion) during the first hour of short-access (ShA; 2 h/day) and long-access (LgA; 12 h/day) oxycodone self-administration in male and female HS rats (N = 542). Females exhibited greater escalation from day 6 of LgA onward (***p < 0.01 vs LgA 1 and ##p < 0.01 vs males, two-way ANOVA with Bonferroni post hoc tests). B Violin plot depicting the number of oxycodone infusions under progressive ratio (PR) testing after ShA and LgA phases in male and female HS rats (N = 542; ***p < 0.01 vs ShA, ##p < 0.01 vs males, two-way ANOVA with Bonferroni post hoc tests). C Development of tolerance to oxycodone’s analgesic effects. Tail withdrawal thresholds (in seconds) measured via the tail immersion test at baseline (BSL), after two oxycodone infusions pre-extended-access self-administration (Oxy-pre-SA), and post- extended-access self-administration (Oxy post-SA) in male and female HS rats (N = 542, ***p < 0.001 vs BSL and ###p < 0.001 vs Oxy-pre-SA, one-way ANOVA with Bonferroni post hoc tests). D Development of withdrawal-induced hyperalgesia in male and female HS rats. Data represent percent change from baseline in paw withdrawal force (g) measured via the von Frey test before and after extended-access oxycodone self-administration (N = 542; ***p < 0.001 vs BSL, paired t-test).
Addiction Index: evaluation of individual differences in addiction-like behaviors
To quantify individual variability in oxycodone addiction-like behaviors, we used an Addiction Index (Fig. 3E) [31, 41,42,43,44,45], integrating four measures: escalation of oxycodone intake under a fixed-ratio (FR) schedule of reinforcement, motivation under a progressive ratio (PR) schedule, tolerance to oxycodone’s analgesic effects, and withdrawal-induced hyperalgesia. Each measure was normalized within cohorts (n = 46–60) and sex using Z-scores. To validate the necessity of this approach, we analyzed the raw, non-normalized total oxycodone intake across cohorts (Fig. S3). A one-way ANOVA revealed significant inter-cohort variability (F(11,540) = 3.254; p = 0.0003), confirming the presence of batch effects. Consequently, within-cohort normalization was utilized to remove these technical confounds, ensuring that the subsequent stratification reflected intrinsic biological phenotype rather than experimental batch variation.
A–D Z-scores for individual measures in the HS rat population (n = 542). A Escalation of oxycodone intake (average of last 3 days of long-access [LgA] self-administration), B motivation (progressive ratio [PR] breakpoint post-LgA, C withdrawal-induced hyperalgesia (percent reduction in von Frey test thresholds), and D tolerance (difference in tail immersion test responses pre- and post-LgA). E Addiction Index, calculated as the mean of the four Z-scores, categorizing rats into quartiles: severe (red), moderate (orange), mild (yellow), and resilient (green). The scatter plot depicts individual rats along the principal analysis, highlighting resilient (green) and severe (red) groups. F Addiction Index distribution for individual rats, showing constituent Z-scores and classifications by vulnerability (resilient vs. severe) and sex (male vs. female).
Z-scores were calculated as (Z = \(\frac{x-\mu }{\sigma }\)), where \(\chi\) is the raw value, \(\mu\) is the mean of the cohort per sex, and \(\sigma\) is the standard deviation of the cohort per sex. We thus obtained an Escalation Index (Fig. 3A), PR Index (Fig. 3B), Tolerance Index (Fig. 3C), and Hyperalgesia Index (Fig. 3D). To quantify the intensification of drug taking relative to initial exposure, daily intake values were normalized against the distribution of the first day LgA phase. Specifically, the Z-score for any given session i (Zi) was calculated using the individual’s daily intake (Xi) relative to the group mean (μday1) and standard deviation (σday1) of the first day of escalation, according to the formula: Zi = (Xi−μday1)/σday1. The final Escalation Index for each subject was defined as the average of these Z-scores calculated over the final three sessions of the self-administration phase. The PR index used the Z-score post-LgA breakpoint. The tolerance index was calculated from the Z-score of the difference in tail immersion test response pre- and post- LgA. The Pain Index reflected the Z-score of the percent reduction in von Frey test withdrawal thresholds during withdrawal relative to baseline. The overall Addiction Index was computed as the mean of these four Z-scores (Fig. 3E). PCA of the four normalized measures identified a first principal component (PC1) with an eigenvalue > 1 that explained ~40% of the total variance, with component loadings ranging from r = 0.19 to 0.86. Detailed examination revealed that PC1 was heavily dominated by self-administration behaviors (escalation: r = 0.86; motivation: r = 0.86), while physiological measures contributed minimally to this component (hyperalgesia: r = 0.19; tolerance: r = 0.19). Consistent with this, the correlation matrix confirmed a moderate association between intake and motivation (r = 0.53) but low correlations between behavioral and physiological measures (r < 0.10). Consequently, utilizing a scoring system based on PC1 weights would result in an index that disproportionately reflects drug-taking behavior while effectively excluding physiological dependence. To avoid this bias and ensure the phenotype reflects a comprehensive addiction syndrome encompassing both drug-seeking and physical dependence (consistent with clinical diagnostic criteria), we rejected the PC1-weighted approach and retained the equal-weighting strategy (simple average of the four Z-scores) for the Addiction Index. Rats were ranked by Addiction Index and divided into four equal quartiles- resilient, mild, moderate, and severe-to classify vulnerability (Fig. 3F).
Oxycodone intake increased across Addiction Index-defined groups (resilient, mild, moderate, severe). Two-way ANOVA (group x session) revealed significant effects of group (F(3, 538) = 75.85; p < 0.0001), session (F(17, 538) = 239.1; p < 0.0001), and their interaction (F(51, 9095) = 18.48; p < 0.0001). To quantify the magnitude of this phenotypic stratification independent of sample size, we calculated the effect size, yielding a partial eta squared (η2p) of 0.30, indicating a large effect. Pairwise comparisons confirmed that each group self-administered significantly more oxycodone than its immediate lower group (resilient < mild < moderate < severe, Fig. 4A). To fully characterize the behavioral diversity inherent in the HS rats, we examined individual acquisition trajectories across the self-administration phase. While the population mean suggests a steady escalation of intake, determining individual performance reveals substantial heterogeneity. Figure 5 displays the daily infusion rates for every subject, stratified by sex and colored by cluster assignment. This granular view confirms that the identified clusters represent robust, distinct behavioral phenotypes. Specifically, “severe” animals (red) consistently exhibit rapid escalation of oxycodone intake, whereas “resilient” animals (green) maintain low, stable intake levels throughout the acquisition period, a pattern observed in both males (Fig. 5A) and females (Fig. 5B). In progressive ratio (PR) testing (Figs. 4B and S2C), two-way ANOVA (group × time) showed significant effects of group (F(3, 538) = 60.04; p < 0.0001), time (F(1, 538) = 404.3; p < 0.0001), and their interaction (F(3, 538) = 38.7; p < 0.0001). To quantify the magnitude of the phenotypic differentiation, we calculated the effect size for the main effect of group, yielding a partial eta squared (η2p) of 0.25. This indicates a large biological effect, confirming that the identified clusters exhibit robust differences in motivational drive. Pairwise comparisons showed that all groups increased breakpoints from short-access (ShA) to long-access (LgA) phases (p < 0.01), with each group showing higher motivation than its immediate lower group (severe > moderate > mild > resilient; p < 0.05).
A Oxycodone infusions during short-access (ShA; 2 h/day) and long-access (LgA:12/day) self-administration in resilient, mild, moderate, and severe groups (***p < 0.001). B Motivation assessed by progressive ratio (PR) testing post- ShA and post-LgA, showing infusions per session (***p < 0.001 vs ShA, #p < 0.05 and ###p < 0.001 vs immediate lower group). C Tolerance to oxycodone’s analgesic effects measured by tail immersion test at baseline (BSL), pre-ShA (15 min post-oxycodone, 150 μg/kg/infusion × 2), and post- LgA (12 h withdrawal, post-oxycodone). Data show tail withdrawal (***p < 0.001 vs BSL and ###p < 0.001 vs Oxy-pre-SA, @p < 0.05 vs immediate lower group). D Withdrawal-induced hyperalgesia assessed by von Frey test, expressed as percent change in paw withdrawal force from baseline at 10–12 h post-LgA (***p < 0.001 vs BSL; #p < 0.05, ##p < 0.01, and ###p < 0.001 vs. immediate lower group). E Linear regression analysis correlating baseline oxycodone-induced analgesia (tail immersion) with the final Addiction Index for all subjects (n = 542). Each dot represents an individual animal. The analysis reveals a highly significant positive correlation (r = 0.2426, p < 0.0001), identifying high initial physiological sensitivity as a risk factor for the development of the severe addiction phenotype.
Longitudinal oxycodone self-administration data for A males and B females. Thin semi-transparent lines represent the daily infusion counts for individual rats, color-coded by their designated cluster (red = severe, orange = moderate, yellow = mild, green = resilient). Thick solid lines with shaded bands represent the Mean ± SEM for each cluster. The visualization highlights the extensive individual variability within the heterogeneous stock (HS) population and confirms that the identified clusters capture distinct acquisition trajectories that are masked by the total population average.
In the tail immersion test, two-way ANOVA (group x time) showed a significant interaction (F(6, 1070) = 22.00; p < 0.0001). Bonferroni post hoc tests demonstrated all groups showed increased analgesia post-oxycodone infusion (150 μg/kg × 2) pre-ShA (p < 0.001 vs. baseline; Fig. 4C). The severe group showed greater analgesia than other groups (p < 0.001, Fig. 4C). To determine if initial sensitivity to the analgesic effects of oxycodone serves as a predictive biomarker for addiction vulnerability, we correlated baseline oxycodone-induced analgesia with the final Addiction Index (Fig. 4E). Linear regression analysis revealed a highly significant positive correlation (r = 0.2426, P < 0.0001). However, the coefficient of determination (R2 = 0.0589) indicates that analgesic sensitivity explains only a small fraction of the total phenotypic variance. After extended access to oxycodone self-administration, moderate and severe animals showed development of tolerance to the analgesic effects of oxycodone (as demonstrated by the decreased response in the tail immersion test after a dose of oxycodone compared to the response pre-self-administration). The magnitude of this physiological adaptation over time was substantial (η2p = 0.37), confirming the robust induction of opioid tolerance in the high-intake groups, perhaps due to greater drug exposure. On the other hand, the resilient group showed a significantly increased response compared to the baseline (pre-self-administration), suggestive of a sensitization of the analgesic effect of oxycodone that was probably due to the low and intermittent level of oxycodone intake during extended access to oxycodone self-administration (Fig. 4C). This effect in the resilient group also extended to the von Frey test. The analysis showed that all the groups showed emergence of hyperalgesia during acute withdrawal from oxycodone (p < 0.001 vs BSL) except for the resilient group, which showed instead a significantly increased paw withdrawal response in the von Frey test after extended access to oxycodone self-administration (p < 0.05 vs BSL). The one-way ANOVA test showed a significant difference between the groups (F(3, 538) = 30.64; p < 0.0001). Calculation of the effect size yielded an eta squared (η2p) of 0.15, indicating a large effect and confirming that withdrawal-induced hyperalgesia is strongly dependent on the addiction phenotype. The Bonferroni post hoc test demonstrated that each of the subgroups (divided based on their Addiction Index) showed increased oxycodone withdrawal-induced hyperalgesia compared to their immediate lower subgroup (resilient < mild < moderate < severe, Fig. 4D).
To confirm that our stratification strategy captures true biological variation rather than arbitrary cutoffs, we performed an unsupervised clustering analysis on the raw behavioral data. We utilized the Bayesian Stochastic Block Model (BSBM) algorithm, which constructs a subject-subject similarity network to infer latent population structures [29, 46]. This unbiased analysis identified four stable clusters (Fig. 6A). We profiled these clusters using a behavioral heatmap (Fig. 6B), which revealed that Cluster 1 displayed a “severe” behavioral profile (high susceptibility), whereas Cluster 4 displayed a “resilient” profile. A Sankey diagram (Fig. 6C) illustrates the correspondence between these unsupervised clusters and our originally defined groups. A Chi-square test confirmed a highly significant association between the two methods (χ2 = 863.1, df = 9, p < 0.0001), validating our stratification criteria.
A Uniform Manifold Approximation and Projection (UMAP) plot visualizing the data structure of the total population (n = 542). Points are colored by the four clusters identified via the unsupervised BSBM algorithm. B Behavioral heatmap profiling the four unsupervised clusters. Rows represent Z-score normalized behavioral features; columns represent the clusters. The color scale indicates deviation from the population mean. The analysis confirms that Cluster 1 aligns with the severe phenotype, while Cluster 4 aligns with the resilient phenotype. C Sankey diagram illustrating the concordance between the original behavioral phenotypes (Left) and the unsupervised BSBM clusters (Right). The flow width represents the number of animals. A Chi-square test indicates a significant dependence between the manual and unsupervised classification methods (χ2 = 863.1, df = 9, p < 0.0001).
Discussion
We conducted a comprehensive behavioral screening of 542 genetically diverse HS rats to characterize oxycodone addiction-like behaviors, including escalation of intake, motivation, tolerance, and withdrawal-induced hyperalgesia. We employed a state-of-the-art model of extended access to intravenous self-administration [47, 48], which induces neuroadaptations observed in clinical populations [49,50,51,52,53], and recapitulates seven DSM-5 criteria for severe opioid use disorder: tolerance [54], withdrawal [55, 56], excessive intake [57], unsuccessful quitting efforts [58, 59], time spent obtaining the drug [60], reduced social/recreational activities [50, 61], and continued use despite adverse consequences [53, 62,63,64]. Escalation of oxycodone intake and increased PR responding are interpreted as compulsive use [26]. Unlike prior studies using smaller cohorts, inbred strains [65], or mouse panels (BXD [66], HMDP [67]) and proxies like locomotion [65] passive drug effects [68], this study leverages the genetic diversity of HS rats, which exhibit high inter-individual variability and low intra-individual variability in oxycodone self-administration, providing a robust platform for identifying specific behavioral phenotypes associated with compulsive oxycodone use.
While distinct addiction-related traits—escalation, motivation, and tolerance—are known to be independently heritable [69], the specific phenotypic architecture of oxycodone vulnerability has remained elusive. By computing a multidimensional Addiction Index, we stratified the population into resilient, mild, moderate, and severe quartiles. Resilient rats exhibited minimal escalation and low motivation, whereas “severe” rats displayed a robust addiction-like phenotype characterized by high intake, profound tolerance, and significant withdrawal-associated hyperalgesia
Our analysis identified a critical dissociation between physiological sensitivity and addiction vulnerability. Interestingly, the “severe” group displayed greater analgesic sensitivity to oxycodone prior to self-administration. However, this group also developed the most substantial tolerance post-escalation. Regression analysis revealed that while initial high analgesic sensitivity is a significant statistical predictor of future vulnerability (p < 0.0001), it explains only a small fraction of the variance (R2 ≈ 6%). This suggests that while initial physiological sensitivity is a risk factor, it is not a standalone fate; the development of the full addiction syndrome requires the recruitment of distinct motivational neurocircuitry.
Furthermore, we observed substantial heterogeneity in withdrawal-induced hyperalgesia. As revealed by our PCA, hyperalgesia loaded weakly on the primary addiction component (r = 0.19) compared to voluntary intake measures (r = 0.86). This indicates that in a genetically diverse population, the severity of physiological withdrawal is not strictly commensurate with the magnitude of drug intake. This uncoupling of physical and motivational symptoms validates our decision to use a composite Addiction Index rather than relying on any single metric. While alternative approaches such as Principal Component Regression might treat physiological adaptations solely as external predictors of intake, our “syndrome-based” model integrates tolerance and hyperalgesia as intrinsic components of the disorder, consistent with their status as core diagnostic criteria in the DSM-5.
Regarding sex differences, female HS rats self-administered significantly more oxycodone than males during the long-access phase, consistent with previous literature [33, 35]. Prior evidence suggests only mild sex differences in oxycodone pharmacokinetics (PK) [70]. However, more granular human studies indicate lower plasma concentrations in women due to distribution volume differences [71,72,73]. Consistent with this complexity, we recently characterized the four inbred founder strains of the HS rat and found that while sex differences in oxycodone pharmacokinetics were present, these peripheral PK profiles did not linearly predict susceptibility to self-administration [74]. Furthermore, recent work assessing a panel of inbred strains demonstrated that the emergence of sex differences in oxycodone self-administration is highly strain-dependent, reinforcing that the magnitude of this effect is modulated by genetic background [75]. This dissociation suggests that the elevated intake in female HS rats is not driven by simple metabolic deficits (e.g., faster clearance). Instead, it likely reflects behavioral compensation to overcome differences in brain oxycodone availability (as hypothesized in our previous work in Wistar rats [33]) or reduced mu-opioid receptor efficacy, necessitating higher intake to achieve comparable central reward magnitude.
Although most individuals initiate oxycodone use via the oral route, oral bioavailability of oxycodone in rats is extremely low (1.2–5.0%; [76]) compared to 60–87% in humans [77], which has led most rat studies to use the intravenous route to ensure reliable brain exposure. Nevertheless, recent oral oxycodone self-administration studies in rats have consistently shown robust intake, with females self-administering significantly more oxycodone than males [78,79,80]. The marked sex differences observed in these oral studies mirror the female-greater intake seen in the current intravenous paradigm, indicating that fundamental sex-dependent vulnerability mechanisms operate independently of administration route.
While ours is the first study to examine extended access oxycodone in HS rats at this scale, similar work using heroin identified three vulnerability clusters, confirming our observation that females are overrepresented in the vulnerable phenotype [28, 29]. Notably, this work extended stratification to the neural level, identifying distinct, sex-specific patterns of Fos activation distinguishing resilience from vulnerability [29]. Together, these studies across different opioids validate the HS rat model as a robust platform for capturing the individual variability necessary to dissect both the behavioral and neuronal mechanisms of addiction.
Finally, this study lays the foundation for future genetic analysis. Previous studies using classical inbred rat strains have established substantial heritability for multiple opioid-related traits, including baseline heroin self-administration [81,82,83], escalation of heroin intake [84], preferred opioid dose [84], acute reactivity to opioid withdrawal [85, 86], antinociceptive effect [87], opioid-induced conditioned place preference [88], proenkephalin levels [81], and m opioid receptor regulation [89]—findings that underscore the genetic underpinnings of opioid use disorder observed in humans. Building on this foundation, the HS rat population used here is optimized for fine-mapping these genetic loci [23, 24]. Ongoing studies are dissecting sex differences in the neurobiological adaptations produced by addiction-like behaviors to oxycodone [90,91,92], and future studies will integrate the behavioral phenotypes characterized here with genotype data to identify gene variants associated with vulnerability and resilience.
Additionally, tissue samples from these behaviorally and genetically characterized rats are available through the oxycodone biobank [43], facilitating the investigation of non-genetic mechanisms, such as epigenetic, transcriptomic [68], and microbiomic factors—that underlie the transition from controlled use to addiction.
References
Administration SAaMHS. In: Administration SAaMHS, editor. NSDUH Series H-46, HHS, Publication No. (SMA) 13-4795. Rockville, MD: Administration SAaMHS; 2013.
Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, et al. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–74.
Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. 2016;374:154–63.
Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ. 2013;346:f2690.
Agrawal A, Verweij KJH, Gillespie NA, Heath AC, Lessov-Schlaggar CN, Martin NG, et al. The genetics of addiction—a translational perspective. Transl Psychiatry. 2012;2:e140.
Ducci F, Goldman D. The genetic basis of addictive disorders. Psychiatr Clin North Am. 2012;35:495–519.
Nielsen DA, Ji F, Yuferov V, Ho A, He C, Ott J, et al. Genome-wide association study identifies genes that may contribute to risk for developing heroin addiction. Psychiatr Genet. 2010;20: https://doi.org/10.1097/YPG.0b013e32833a2106.
Erzurumluoglu AM, Liu M, Jackson VE, Barnes DR, Datta G, Melbourne CA, et al. Meta-analysis of up to 622,409 individuals identifies 40 novel smoking behaviour associated genetic loci. Mol Psychiatry. 2020;25:2392–409.
Walters RK, Polimanti R, Johnson EC, McClintick JN, Adams MJ, Adkins AE, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21:1656–69.
Zhou H, Sealock JM, Sanchez-Roige S, Clarke TK, Levey DF, Cheng Z, et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat Neurosci. 2020;23:809–18.
Sanchez-Roige S, Fontanillas P, Elson SL, 23andMe Research Team, Gray JC, de Wit H, et al. Genome-wide association study of alcohol use disorder identification test (AUDIT) scores in 20 328 research participants of European ancestry. Addict Biol. 2019;24:121–31.
Sanchez-Roige S, Palmer AA, Fontanillas P, Elson SL, 23andMe Research Team, the Substance Use Disorder Working Group of the Psychiatric Genomics Consortium, Adams MJ, et al. Genome-Wide Association Study Meta-Analysis of the Alcohol Use Disorders Identification Test (AUDIT) in two population-based cohorts. Am J Psychiatry. 2019;176:107–18.
Hatoum AS, Colbert SMC, Johnson EC, Huggett SB, Deak JD, Pathak G, et al. Multivariate genome-wide association meta-analysis of over 1 million subjects identifies loci underlying multiple substance use disorders. Nat Ment Health. 2023;1:210–23.
Karlsson Linner R, Mallard TT, Barr PB, Sanchez-Roige S, Madole JW, Driver MN, et al. Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self-regulation and addiction. Nat Neurosci. 2021;24:1367–76.
Deak JD, Zhou H, Galimberti M, Levey DF, Wendt FR, Sanchez-Roige S, et al. Genome-wide association study in individuals of European and African ancestry and multi-trait analysis of opioid use disorder identifies 19 independent genome-wide significant risk loci. Mol Psychiatry. 2022;27:3970–79.
Kember RL, Vickers-Smith R, Xu H, Toikumo S, Niarchou M, Zhou H, et al. Cross-ancestry meta-analysis of opioid use disorder uncovers novel loci with predominant effects in brain regions associated with addiction. Nat Neurosci. 2022;25:1279–87.
Polimanti R, Walters RK, Johnson EC, McClintick JN, Adkins AE, Adkins DE, et al. Leveraging genome-wide data to investigate differences between opioid use vs. opioid dependence in 41,176 individuals from the Psychiatric Genomics Consortium. Mol Psychiatry. 2020;25:1673–87.
Sanchez-Roige S, Fontanillas P, Jennings MV, Bianchi SB, Huang Y, Hatoum AS, et al. Genome-wide association study of problematic opioid prescription use in 132,113 23andMe research participants of European ancestry. Mol Psychiatry. 2021;26:6209–17.
Song W, Kossowsky J, Torous J, Chen CY, Huang H, Mukamal KJ, et al. Genome-wide association analysis of opioid use disorder: a novel approach using clinical data. Drug Alcohol Depend. 2020;217:108276.
Zhou H, Rentsch CT, Cheng Z, Kember RL, Nunez YZ, Sherva RM, et al. Association of OPRM1 functional coding variant with opioid use disorder: a genome-wide association study. JAMA Psychiatry. 2020;77:1072–80.
Hart AB, de Wit H, Palmer AA. Candidate gene studies of a promising intermediate phenotype: failure to replicate. Neuropsychopharmacology. 2013;38:802–16.
Klepstad P, Fladvad T, Skorpen F, Bjordal K, Caraceni A, Dale O, et al. Influence from genetic variability on opioid use for cancer pain: a European genetic association study of 2294 cancer pain patients. Pain. 2011;152:1139–45.
Chitre AS, Polesskaya O, Holl K, Gao J, Cheng R, Bimschleger H, et al. Genome-wide association study in 3,173 outbred rats identifies multiple loci for body weight, adiposity, and fasting glucose. Obesity. 2020;28:1964–73.
Solberg Woods LC, Palmer AA. Using heterogeneous stocks for fine-mapping genetically complex traits. Methods Mol Biol. 2019;2018:233–47.
Zhang Y, Mayer-Blackwell B, Schlussman SD, Randesi M, Butelman ER, Ho A, et al. Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6J mice. Psychopharmacology. 2014;231:1277–87.
Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, Koob GF. Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology. 2015;40:421–28.
Nielsen LM, Olesen AE, Branford R, Christrup LL, Sato H, Drewes AM. Association between human pain-related genotypes and variability in opioid analgesia: an updated review. Pain Pract. 2015;15:580–94.
Kuhn BN, Cannella N, Chitre AS, Nguyen KH, Cohen K, Chen D, et al. Genome-wide association study reveals multiple loci for nociception and opioid consumption behaviors associated with heroin vulnerability in outbred rats. Mol Psychiatry. 2025;30:3363–75.
Kuhn BN, Cannella N, Crow AD, Lunerti V, Gupta A, Walterhouse SJ, et al. Distinct behavioral profiles and neuronal correlates of heroin vulnerability versus resiliency in a multi-symptomatic model of heroin use disorder in rats. Am J Psychiatry. 2025;182:198–208.
Bush WS, Moore JH. Chapter 11: Genome-wide association studies. PLoS Comput Biol. 2012;8:e1002822.
Kallupi M, Carrette LLG, Kononoff J, Solberg Woods LC, Palmer AA, Schweitzer P, et al. Nociceptin attenuates the escalation of oxycodone self-administration by normalizing CeA-GABA transmission in highly addicted rats. Proc Natl Acad Sci USA. 2020;117:2140–48.
Hansen C, Spuhler K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol Clin Exp Res. 1984;8:477–9.
Kimbrough A, Kononoff J, Simpson S, Kallupi M, Sedighim S, Palomino K, et al. Oxycodone self-administration and withdrawal behaviors in male and female Wistar rats. Psychopharmacology. 2020;237:1545–55.
Wade CL, Kallupi M, Hernandez DO, Breysse E, de Guglielmo G, Crawford E, et al. High-frequency stimulation of the subthalamic nucleus blocks compulsive-like re-escalation of heroin taking in rats. Neuropsychopharmacology. 2017;42:1850–59.
Mavrikaki M, Pravetoni M, Page S, Potter D, Chartoff E. Oxycodone self-administration in male and female rats. Psychopharmacology. 2017;234:977–87.
de Guglielmo G, Kallupi M, Sedighim S, Newman AH, George O. Dopamine D(3) receptor antagonism reverses the escalation of oxycodone self-administration and decreases withdrawal-induced hyperalgesia and irritability-like behavior in oxycodone-dependent heterogeneous stock rats. Front Behav Neurosci. 2019;13:292.
Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63.
Smith LC, Kallupi M, Tieu L, Shankar K, Jaquish A, Barr J, et al. Validation of a nicotine vapor self-administration model in rats with relevance to electronic cigarette use. Neuropsychopharmacology. 2020;45:1909–19.
Smith LC, Tieu L, Suhandynata RT, Boomhower B, Hoffman M, Sepulveda Y, et al. Cannabidiol reduces withdrawal symptoms in nicotine-dependent rats. Psychopharmacology. 2021;238:2201–11.
de Guglielmo G, Kallupi M, Scuppa G, Stopponi S, Demopulos G, Gaitanaris G, et al. Analgesic tolerance to morphine is regulated by PPARgamma. Br J Pharmacol. 2014;171:5407–16.
Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–7.
Belin D, Balado E, Piazza PV, Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65:863–8.
Carrette LL, de Guglielmo G, Kallupi M, Maturin L, Brennan M, Boomhower B, et al. The cocaine and oxycodone biobanks, two repositories from genetically diverse and behaviorally characterized rats for the study of addiction. eNeuro. 2021;8:ENEURO.0033-21.2021.
Sedighim S, Carrette LLG, Venniro M, Shaham Y, de Guglielmo G, George O. Individual differences in addiction-like behaviors and choice between cocaine versus food in Heterogeneous Stock rats. Psychopharmacology. 2021;238:3423–33.
de Guglielmo G, Carrette L, Kallupi M, Brennan M, Boomhower B, Maturin L, et al. Large-scale characterization of cocaine addiction-like behaviors reveals that escalation of intake, aversion-resistant responding, and breaking-points are highly correlated measures of the same construct. eLife. 2024;12:RP90422.
Allen C, Kuhn BN, Cannella N, Crow AD, Roberts AT, Lunerti V, et al. Network-based discovery of opioid use vulnerability in rats using the Bayesian Stochastic Block Model. Front Psychiatry. 2021;12:745468.
Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol. 2013;24:356–62.
George O, Koob GF, Vendruscolo LF. Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology. 2014;231:3911–7.
Briand LA, Flagel SB, Seeman P, Robinson TE. Cocaine self-administration produces a persistent increase in dopamine D2 High receptors. Eur Neuropsychopharmacology. 2008;18:551–6.
George O, Mandyam CD, Wee S, Koob GF. Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology. 2008;33:2474–82.
George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, et al. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc Natl Acad Sci USA. 2012;109:18156–61.
Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, et al. Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Arch Gen Psychiatry. 1990;47:325–30.
Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW Jr., Logrip ML, et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–71.
Ben-Shahar O, Moscarello JM, Ettenberg A. One hour, but not six hours, of daily access to self-administered cocaine results in elevated levels of the dopamine transporter. Brain Res. 2006;1095:148–53.
Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–6.
Vendruscolo LF, Schlosburg JE, Misra KK, Chen SA, Greenwell TN, Koob GF. Escalation patterns of varying periods of heroin access. Pharmacol Biochem Behav. 2011;98:570–4.
Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300.
Ahmed SH, Cador M. Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology. 2006;31:563–71.
Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PloS one. 2007;2:e698.
Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacology. 2008;18:303–11.
Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology. 2013;38:1209–20.
Seif T, Chang SJ, Simms JA, Gibb SL, Dadgar J, Chen BT, et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat Neurosci. 2013;16:1094–100.
Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–9.
Xue Y, Steketee JD, Sun W. Inactivation of the central nucleus of the amygdala reduces the effect of punishment on cocaine self-administration in rats. Eur J Neurosci. 2012;35:775–83.
Zhou Z, Blandino P, Yuan Q, Shen PH, Hodgkinson CA, Virkkunen M, et al. Exploratory locomotion, a predictor of addiction vulnerability, is oligogenic in rats selected for this phenotype. Proc Natl Acad Sci USA. 2019;116:13107–15.
Dickson PE, Miller MM, Calton MA, Bubier JA, Cook MN, Goldowitz D, et al. Systems genetics of intravenous cocaine self-administration in the BXD recombinant inbred mouse panel. Psychopharmacology. 2016;233:701–14.
Bagley JR, Arshad HK, Smith DJ, Jentsch JD. Extreme phenotypic diversity in operant responding for an intravenous cocaine or saline infusion in the Hybrid Mouse Diversity Panel. Addict Biol. 2022;27:e13162.
Vorspan F, Icick R, Mekdad N, Courtin C, Bloch V, Bellivier F, et al. Translational study of the whole transcriptome in rats and genetic polymorphisms in humans identifies LRP1B and VPS13A as key genes involved in tolerance to cocaine-induced motor disturbances. Transl Psychiatry. 2020;10:381.
Eid M, Pullmann D, LI H, Thomas A, Jhou TC. Multiple elements of addiction vulnerability are independently heritable in rats. Biorxiv [Preprint]. 2019. Available from: https://doi.org/10.1101/566018
Graziani M, Nistico R. Gender difference in prescription opioid abuse: a focus on oxycodone and hydrocodone. Pharmacol Res. 2016;108:31–8.
Andreassen TN, Klepstad P, Davies A, Bjordal K, Lundstrom S, Kaasa S, et al. Influences on the pharmacokinetics of oxycodone: a multicentre cross-sectional study in 439 adult cancer patients. Eur J Clin Pharmacol. 2011;67:493–506.
Kaiko RF, Benziger DP, Fitzmartin RD, Burke BE, Reder RF, Goldenheim PD. Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther. 1996;59:52–61.
Prozesky L. Heartwater. The morphology of Cowdria ruminantium and its staining characteristics in the vertebrate host and in vitro. Onderstepoort J Vet Res. 1987;54:173–6.
Doyle MR, Martinez AR, Qiao R, Dirik S, Di Ottavio F, Pascasio G, et al. Strain and sex-related behavioral variability of oxycodone dependence in rats. Neuropharmacology. 2023;237:109635.
Duffy EP, Ward JO, Hale LH, Brown KT, Kwilasz AJ, Mehrhoff EA, et al. Sex and genetic background influence intravenous oxycodone self-administration in the hybrid rat diversity panel. Front Psychiatry. 2024;15:1505898.
Chan S, Edwards SR, Wyse BD, Smith MT. Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol. 2008;35:295–302.
Huddart R, Clarke M, Altman RB, Klein TE. PharmGKB summary: oxycodone pathway, pharmacokinetics. Pharmacogenet Genom. 2018;28:230–37.
LaRocco K, Villiamma P, Hill J, Russell MA, DiLeone RJ, Groman SM. Disruptions in reward-guided decision-making functions are predictive of greater oral oxycodone self-administration in male and female rats. Biol Psychiatry Glob Open Sci. 2025;5:100450.
Sharp BM, Leng S, Huang J, Jones C, Williams RW, Chen H. Inbred rat heredity and sex affect oral oxycodone self-administration and augmented intake in long sessions: correlations with anxiety and novelty-seeking. PLoS ONE. 2025;20:e0314777.
Zanni G, DeSalle MJ, Deutsch HM, Barr GA, Eisch AJ. Female and male rats readily consume and prefer oxycodone to water in a chronic, continuous access, two-bottle oral voluntary paradigm. Neuropharmacology. 2020;167:107978.
Martin S, Manzanares J, Corchero J, Garcia-Lecumberri C, Crespo JA, Fuentes JA, et al. Differential basal proenkephalin gene expression in dorsal striatum and nucleus accumbens, and vulnerability to morphine self-administration in Fischer 344 and Lewis rats. Brain Res. 1999;821:350–5.
Garcia-Lecumberri C, Torres I, Martin S, Crespo JA, Miguens M, Nicanor C, et al. Strain differences in the dose-response relationship for morphine self-administration and impulsive choice between Lewis and Fischer 344 rats. J Psychopharmacol. 2011;25:783–91.
Sanchez-Cardoso P, Higuera-Matas A, Martin S, Miguens M, Del Olmo N, Garcia-Lecumberri C, et al. Strain differences between Lewis and Fischer 344 rats in the modulation of dopaminergic receptors after morphine self-administration and during extinction. Neuropharmacology. 2009;57:8–17.
Picetti R, Caccavo JA, Ho A, Kreek MJ. Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology. 2012;220:163–72.
Cobuzzi JL, Riley AL. Spontaneous withdrawal in opiate-dependent Fischer 344, Lewis and Sprague-Dawley rats. Pharmacol Biochem Behav. 2011;98:28–34.
Kest B, Smith SB, Schorscher-Petcu A, Austin JS, Ritchie J, Klein G, et al. Gnao1 (G alphaO protein) is a likely genetic contributor to variation in physical dependence on opioids in mice. Neuroscience. 2009;162:1255–64.
Sudakov SK, Borisova EV, Lyupina YV. Influence of inheritance and fostering on sensitivity to effects of morphine on nociception and locomotor activity in two inbred rat strains. Neuropharmacology. 1996;35:1131–4.
Guitart X, Beitner-Johnson D, Marby DW, Kosten TA, Nestler EJ. Fischer and Lewis rat strains differ in basal levels of neurofilament proteins and their regulation by chronic morphine in the mesolimbic dopamine system. Synapse. 1992;12:242–53.
Petruzzi R, Ferraro TN, Kurschner VC, Golden GT, Berrettini WH. The effects of repeated morphine exposure on mu opioid receptor number and affinity in C57BL/6J and DBA/2J mice. Life Sci. 1997;61:2057–64.
Delorme TC, Sambare S, Williams BR, Gamble MC, Solberg Woods LC, Maturin L, et al. Sex-specific transcriptional signatures of oxycodone persist during withdrawal and abstinence in the suprachiasmatic nucleus of heterogeneous stock rats. bioRxiv [Preprint]. 2025. Available from: https://doi.org/10.1101/2025.04.29.651331
Shelton MA, Horan N, Xue X, Maturin L, Eacret D, Michaud J, et al. Sex-Specific concordance of striatal transcriptional signatures of opioid addiction in human and rodent brains. Biol Psychiatry Glob Open Sci. 2025;5:100476.
Vu T, Godbole S, Carrette LLG, Maturin L, George O, Saba LM, et al. Identification of plasma metabolites responding to oxycodone exposure in rats. Metabolites. 2025;15:95.
Funding
This work was supported by National Institutes of Health grant U01DA044451 from the National Institute on Drug Abuse.
Author information
Authors and Affiliations
Contributions
MK and GdG conceptualized and designed the study, performed data analysis, and drafted the initial manuscript. OG designed the study, revised the manuscript, and provided overall study supervision. OG and AP secured funding. LSW supplied heterogeneous stock (HS) rats. AP and LSW contributed to manuscript preparation. LM facilitated organ collection and managed the biobank organization. BP, AC, and OP provided HS rats and assisted with data acquisition. LC, SS, JK, AK, LCS, KS, AA, DC, MB, LT, SS, BB, MKJF, AM, CC, SD, NV, PS, SBZ, ES, SP, AM, DO, BS, JL, and AV contributed to data acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kallupi, M., de Guglielmo, G., Carrette, L.L.G. et al. Large-scale behavioral characterization of oxycodone self-administration in heterogeneous stock rats reveals initial analgesic effects are associated with addiction-like behaviors. Neuropsychopharmacol. (2026). https://doi.org/10.1038/s41386-026-02348-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41386-026-02348-8