Abstract
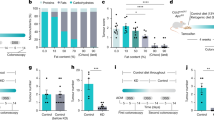
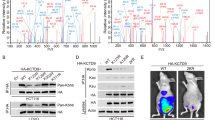
Colorectal cancer (CRC) ranks third in incidence and second in mortality worldwide. Metabolic disorders are known to be closely associated with CRC. Functional metabolomics aims to translate metabolomics-derived biomarkers to disease mechanisms. Previous work based on untargeted liquid chromatography identified 30 differential metabolites of CRC. Among them, only β-hydroxybutyrate (BHB) was elevated in CRC. Here, we first confirm the increased level of β-hydroxybutyrate by targeted metabolomic analysis using an independent cohort of 400 serum samples by UPLC-QQQ-MS/MS analysis. Using appropriate cell and animal models, we find that treatment with pathological levels of β-hydroxybutyrate expedites CRC proliferation and metastasis. Out of four major rate-limiting enzymes of ketolysis, only acetyl-coenzyme A acetyltransferase1 (ACAT1) expression is increased in paired human CRC tissues. These findings suggest probable clinical relevance for the functional implications of β-hydroxybutyrate in CRC. We demonstrate that β-hydroxybutyrate may exert its tumorigenic effects via regulation of ACAT1, due to induction of downstream isocitrate dehydrogenase1 (IDH1) acetylation. Genetic silencing of ACAT1 significantly suppresses the progression of CRC and abrogates the effects of β-hydroxybutyrate both in vitro and in vivo. Overall, this study suggests that targeting β-hydroxybutyrate and its major rate-limiting enzyme ACAT1 may provide a new avenue for therapeutic intervention in CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data associated with this study are presented in the paper or the Supplementary Material. The materials that support the findings of this study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel R, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80.
Manna SK, Tanaka N, Krausz KW, Haznadar M, Xue X, Matsubara T, et al. Biomarkers of coordinate metabolic reprogramming in colorectal tumors in mice and humans. Gastroenterology. 2014;146:1313–24.
La Vecchia S, Sebastian C. Metabolic pathways regulating colorectal cancer initiation and progression. Semin Cell Dev Biol. 2020;98:63–70.
Li J, Li J, Wang H, Qi LW, Zhu Y, Lai M. Tyrosine and glutamine-leucine are metabolic markers of early-stage colorectal cancers. Gastroenterology. 2019;157:257–9.e255.
Garcia E, Shalaurova I, Matyus SP, Oskardmay DN, Otvos JD, Dullaart RPF, et al. Ketone bodies are mildly elevated in subjects with type 2 diabetes mellitus and are inversely associated with insulin resistance as measured by the lipoprotein insulin resistance index. J Clin Med. 2020;9:321.
Yamane N, Tsuda T, Nose K, Yamamoto A, Ishiguro H, Kondo T. Relationship between skin acetone and blood beta-hydroxybutyrate concentrations in diabetes. Clin Chim Acta. 2006;365:325–9.
Dehal AN, Newton CC, Jacobs EJ, Patel AV, Gapstur SM, Campbell PT. Impact of diabetes mellitus and insulin use on survival after colorectal cancer diagnosis: the Cancer Prevention Study-II Nutrition Cohort. J Clin Oncol. 2012;30:53–59.
Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607.
Pang Y, Kartsonaki C, Guo Y, Chen Y, Yang L, Bian Z, et al. Diabetes, plasma glucose and incidence of colorectal cancer in Chinese adults: a prospective study of 0.5 million people. J Epidemiol Community Health. 2018;0:1–7.
Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25:42–52.
Papalazarou V, Maddocks ODK. Supply and demand: cellular nutrient uptake and exchange in cancer. Mol Cell. 2021;81:3731–48.
Jiang L, Shestov AA, Swain P, Yang C, Parker SJ, Wang QA, et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature. 2016;532:255–8.
Kuo CC, Ling HH, Chiang MC, Chung CH, Lee WY, Chu CY, et al. Metastatic colorectal cancer rewrites metabolic program through a Glut3-YAP-dependent signaling circuit. Theranostics. 2019;9:2526–40.
Loo JM, Scherl A, Nguyen A, Man FY, Weinberg E, Zeng Z, et al. Extracellular metabolic energetics can promote cancer progression. Cell. 2015;160:393–406.
Bu P, Chen KY, Xiang K, Johnson C, Crown SB, Rakhilin N, et al. Aldolase B-mediated fructose metabolism drives metabolic reprogramming of colon cancer liver metastasis. Cell Metab. 2018;27:1249–62. e1244
Wang YN, Zeng ZL, Lu J, Wang Y, Liu ZX, He MM, et al. CPT1A-mediated fatty acid oxidation promotes colorectal cancer cell metastasis by inhibiting anoikis. Oncogene. 2018;37:6025–40.
Puchalska P, Crawford PA. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–84.
Zhu Y, Gu L, Lin X, Liu C, Lu B, Cui K, et al. Dynamic regulation of ME1 phosphorylation and acetylation affects lipid metabolism and colorectal tumorigenesis. Mol Cell. 2020;77:138–49.e135.
Fan J, Shan C, Kang HB, Elf S, Xie J, Tucker M, et al. Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol Cell. 2014;53:534–48.
Gu L, Zhu Y, Lin X, Tan X, Lu B, Li Y. Stabilization of FASN by ACAT1-mediated GNPAT acetylation promotes lipid metabolism and hepatocarcinogenesis. Oncogene. 2020;39:2437–49.
Wang B, Ye Y, Yang X, Liu B, Wang Z, Chen S, et al. SIRT2-dependent IDH1 deacetylation inhibits colorectal cancer and liver metastases. EMBO Rep. 2020;21:e48183.
Huang CK, Chang PH, Kuo WH, Chen CL, Jeng YM, Chang KJ, et al. Adipocytes promote malignant growth of breast tumours with monocarboxylate transporter 2 expression via beta-hydroxybutyrate. Nat Commun. 2017;8:14706.
Nagao M, Toh R, Irino Y, Mori T, Nakajima H, Hara T, et al. beta-Hydroxybutyrate elevation as a compensatory response against oxidative stress in cardiomyocytes. Biochem Biophys Res Commun. 2016;475:322–8.
Nonaka Y, Takagi T, Inai M, Nishimura S, Urashima S, Honda K, et al. Lauric acid stimulates ketone body production in the KT-5 astrocyte cell line. J Oleo Sci. 2016;65:693–9.
Zhang D, Yang H, Kong X, Wang K, Mao X, Yan X, et al. Proteomics analysis reveals diabetic kidney as a ketogenic organ in type 2 diabetes. Am J Physiol Endocrinol Metab. 2011;300:E287–295.
Song J-P, Chen L, Chen X, Ren J, Zhang N-N, Tirasawasdichai T, et al. Elevated plasma β-hydroxybutyrate predicts adverse outcomes and disease progression in patients with arrhythmogenic cardiomyopathy. Sci Transl Med. 2020;12:eaay8329.
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203.
Wu JY, Huang TW, Hsieh YT, Wang YF, Yen CC, Lee GL, et al. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol Cell. 2020;77:213–27.e215.
Yan X, Liu XY, Zhang D, Zhang YD, Li ZH, Liu X, et al. Construction of a sustainable 3-hydroxybutyrate-producing probiotic Escherichia coli for treatment of colitis. Cell Mol Immunol. 2021;18:2344–57.
Wishart DS. Metabolomics for investigating physiological and pathophysiological processes. Physiol Rev. 2019;99:1819–75.
Liang L, Sun F, Wang H, Hu Z. Metabolomics, metabolic flux analysis and cancer pharmacology. Pharmacol Ther. 2021;224:107827.
Schmidt DR, Patel R, Kirsch DG, Lewis CA, Vander Heiden MG, Locasale JW. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J Clin. 2021;71:333–58.
Gao R, Wu C, Zhu Y, Kong C, Zhu Y, Gao Y, et al. Integrated analysis of colorectal cancer reveals cross-cohort gut microbial signatures and associated serum metabolites. Gastroenterology. 2022;163:1024–37.e1029.
Chen F, Dai X, Zhou C-C, Li K-X, Zhang Y-J, Lou X-Y, et al. Integrated analysis of the faecal metagenome and serum metabolome reveals the role of gut microbiome-associated metabolites in the detection of colorectal cancer and adenoma. Gut. 2021;71:1315–25.
Ghini V, Abuja PM, Polasek O, Kozera L, Laiho P, Anton G, et al. Impact of the pre-examination phase on multicenter metabolomic studies. N Biotechnol. 2022;68:37–47.
Gertsman I, Barshop BA. Promises and pitfalls of untargeted metabolomics. J Inherit Metab Dis. 2018;41:355–66.
Han T, Zhan W, Gan M, Liu F, Yu B, Chin YE, et al. Phosphorylation of glutaminase by PKCepsilon is essential for its enzymatic activity and critically contributes to tumorigenesis. Cell Res. 2018;28:655–69.
Jaworski DM, Namboodiri AM, Moffett JR. Acetate as a metabolic and epigenetic modifier of cancer therapy. J Cell Biochem. 2016;117:574–88.
Sullivan LB, Gui DY, Hosios AM, Bush LN, Freinkman E, Vander Heiden MG. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 2015;162:552–63.
Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–64.
Zhang H, Chang Z, Qin LN, Liang B, Han JX, Qiao KL, et al. MTA2 triggered R-loop trans-regulates BDH1-mediated beta-hydroxybutyrylation and potentiates propagation of hepatocellular carcinoma stem cells. Signal Transduct Target Ther. 2021;6:135.
Cheng CW, Biton M, Haber AL, Gunduz N, Eng G, Gaynor LT, et al. Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell. 2019;178:1115–31.e1115.
Labanca E, Bizzotto J, Sanchis P, Anselmino N, Yang J, Shepherd PDA, et al. Prostate cancer castrate resistant progression usage of non-canonical androgen receptor signaling and ketone body fuel. Oncogene. 2021;40:6284–98.
Saraon P, Trudel D, Kron K, Dmitromanolakis A, Trachtenberg J, Bapat B, et al. Evaluation and prognostic significance of ACAT1 as a marker of prostate cancer progression. Prostate. 2014;74:372–80.
Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Ketone body utilization drives tumor growth and metastasis. Cell Cycle. 2012;11:3964–71.
Chen D, Xia S, Zhang R, Li Y, Famulare CA, Fan H, et al. Lysine acetylation restricts mutant IDH2 activity to optimize transformation in AML cells. Mol Cell. 2021;81:3833–47.e3811.
Huang LiT, Wang L, Zhang L, Yan R, Li K, et al. Hepatocellular carcinoma redirects to ketolysis for progression under nutrition deprivation stress. Cell Res. 2016;26:1112–30.
Irrazabal T, Belcheva A, Girardin SE, Martin A, Philpott DJ. The multifaceted role of the intestinal microbiota in colon cancer. Mol Cell. 2014;54:309–20.
Garrett WS. The gut microbiota and colon cancer. Science. 2019;364:1133–5.
Ternes D, Karta J, Tsenkova M, Wilmes P, Haan S, Letellier E. Microbiome in colorectal cancer: how to get from meta-omics to mechanism? Trends Microbiol. 2020;28:401–23.
Acknowledgements
We thank Xiaodong Wen from the Cellular and Molecular Biology Center of China Pharmaceutical University for advice and laboratory support. We are thankful for the grant for financial support from the National Natural Science Foundation of China (Grant Nos. 82002607 and 82072629), Key Research and Development Program of Shaanxi Province (Program No. 2022SF-215), the Open Project of State Key Laboratory of Natural Medicines, No. SKLNMKF202205 and CAMS Innovation Fund for Medical Sciences (CIFMS, 2019-I2M-5-044).
Author information
Authors and Affiliations
Contributions
MDL and JKL conceived the original idea and designed the study. TXM contributed to the experiments and analyzed the data. FJQ and MDZ performed the experiments, in part, and analyzed the data. TXM prepared the draft of the study and revised it by MDL, JKL and JL. All authors agreed with the conclusion and approved the final study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mao, T., Qin, F., Zhang, M. et al. Elevated serum β-hydroxybutyrate, a circulating ketone metabolite, accelerates colorectal cancer proliferation and metastasis via ACAT1. Oncogene 42, 1889–1899 (2023). https://doi.org/10.1038/s41388-023-02700-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-023-02700-y
This article is cited by
-
Pre-diagnostic circulating untargeted metabolomics and risk of overall and clinically significant prostate cancer: a systematic review and meta-analysis
British Journal of Cancer (2026)
-
Circulating proteins and metabolites panel for noninvasive preoperative diagnosis of epithelial ovarian cancer
BMC Medicine (2025)
-
Integrative insights into the role of CAV1 in ketogenic diet and ferroptosis in pancreatic cancer
Cell Death Discovery (2025)
-
Dietary and metabolic effects on intestinal stem cells in health and disease
Nature Reviews Gastroenterology & Hepatology (2025)
-
Differential effects of β-hydroxybutyrate and α-ketoglutarate on HCT-116 colorectal cancer cell viability under normoxic and hypoxic low-glucose conditions: exploring the role of SRC, HIF1α, ACAT1, and SIRT2 genes
Molecular Genetics and Genomics (2025)