Abstract
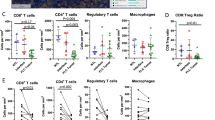
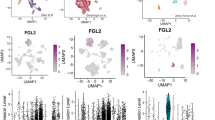
Interleukin-2 (IL2) treatment has been explored as a potent immunotherapy agent, particularly for cancers, due to its ability to stimulate T cell proliferation and activity. However, significant challenges and limitations are associated with IL2 treatment, including its short half-life, systemic toxicity and side effects, and limited efficacy in solid tumors. In this study, we deployed an attenuated Salmonella Gallinarum (SG), an avian-specific pathogen capable of targeting tumor tissue, to express and secrete the IL2 using a bacterial flagellum type 3 secretion system (T3SS). Since the T3SS is used for the secretion of flagellin monomers (FliC), DNA of the human IL2 gene was fused to the SG fliC gene so that the fusion proteins would be exported together. A superb anti-cancer effect was observed when the SG expressing and secreting the FliC-IL2 fusion protein was injected into a syngeneic tumor mouse model with CT26 colorectal cancer via the tail vein. Within the fusion protein, the FliC moiety led to a selective increase in MHCIIhighCD206- M1-like macrophages, while the IL2 moiety promoted selective expansion of cytotoxic CD8+ T cells and NK cells, without expanding CD4+FoxP3+ regulatory T cells in the tumor microenvironment (TME). It was concluded that the local delivery of IL2 within the TME by cancer-targeting SG could overcome the limitations associated with IL2-based cancer immunotherapy.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are included in the manuscript or available from corresponding authors upon reasonable request.
References
Chien T, Doshi A, Danino T. Advances in bacterial cancer therapies using synthetic biology. Curr Opin Syst Biol. 2017;5:1–8.
Raman V, Deshpande CP, Khanduja S, Howell LM, Van Dessel N, Forbes NS. Build-a-bug workshop: Using microbial-host interactions and synthetic biology tools to create cancer therapies. Cell Host Microbe. 2023;31:1574–92.
Zhu D, Pan W, Li H, Hua J, Zhang C, Zhao K. Innovative applications of bacteria and their derivatives in targeted tumor therapy. ACS nano. 2025;19:5077–109.
Macnab RM. How bacteria assemble flagella. Annu Rev Microbiol. 2003;57:77–100.
Macnab RM. Type III flagellar protein export and flagellar assembly. Bba-Mol Cell Res. 2004;1694:207–17.
Bonifield HR, Hughes KT. Flagellar phase variation in Salmonella enterica is mediated by a posttranscriptional control mechanism. J Bacteriol. 2003;185:3567–74.
Chevance FFV, Hughes KT. Coordinating assembly of a bacterial macromolecular machine. Nat Rev Microbiol. 2008;6:455–65.
Singer HM, Erhardt M, Hughes KT. Comparative analysis of the secretion capability of early and late flagellar type III secretion substrates. Mol Microbiol. 2014;93:505–20.
Végh BM, Gál P, Dobó J, Závodszky P, Vonderviszt F. Localization of the flagellum-specific secretion signal in Salmonella flagellin. Biochem Bioph Res Co. 2006;345:93–8.
Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: Bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–5.
Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–103.
Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Barrett SLR, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol. 2003;4:1247–53.
Schomburg A, Menzel T, Körfer A, Heer G, Dallmann I, Kirchner H, et al. In vivo and ex vivo antitumor activity in patients receiving low-dose subcutaneous recombinant interleukin-2. Natural Immun. 1992;11:133–43.
Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451–8.
Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2 - Identification of the antigens mediating response. Ann Surg. 1998;228:307–17.
Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16.
Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–90.
Leonard WJ, Lin JX, O’Shea JJ. The γc family of cytokines: basic biology to therapeutic ramifications. Immunity. 2019;50:832–50.
Takeshita T, Asao H, Ohtani K, Ishii N, Kumaki S, Tanaka N, et al. Cloning of the γ chain of the human IL-2 receptor. Science. 1992;257:379–82.
Taniguchi T. Minami Y. The IL-2IL-2 receptor system: a current overview. Cell. 1993;73:5–8.
Grant AJ, Roessler E, Ju G, Tsudo M, Sugamura K, Waldmann TA. The interleukin 2 receptor (IL-2R): the IL-2R alpha subunit alters the function of the IL-2R beta subunit to enhance IL-2 binding and signaling by mechanisms that do not require binding of IL-2 to IL-2R alpha subunit. Proc Natl Acad Sci. 1992;89:2165–9.
Nakamura Y, Russell SM, Mess SA, Friedmann M, Erdos M, Francois C, et al. Heterodimerization of the IL-2 receptor β-and γ-chain cytoplasmic domains is required for signalling. Nature. 1994;369:330–3.
Nelson BH, Lord JD, Greenberg PD. Cytoplasmic domains of the interleukin-2 receptor β and γ chains mediate the signal for T-cell proliferation. Nature. 1994;369:333–6.
Mitra S, Leonard WJ. Biology of IL-2 and its therapeutic modulation: mechanisms and strategies. J Leukoc Biol. 2018;103:643–55.
Hernandez R, Poder J, LaPorte KM, Malek TR. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. 2022;22:614–28.
Donohue JH, Rosenberg S. The fate of interleukin-2 after in vivo administration. J Immunol. 1983;130:2203–8.
Culliton B. FDA panel backs interleukin-2. Nature. 1992;355:287.
Rosenberg SA. Interleukin 2 for patients with renal cancer. Nat Clin Pr Oncol. 2007;4:497.
Li Y, Strick-Marchand H, Lim AI, Ren JZ, Masse-Ranson G, Li D, et al. Regulatory T cells control toxicity in a humanized model of IL-2 therapy. Nat Commun. 2017;8:1762.
Mott HR, Baines BS, Hall RM, Cooke RM, Driscoll PC, Weir MP, et al. The solution structure of the F42a mutant of human interleukin-2. J Mol Biol. 1995;247:979–94.
Levin AM, Bates DL, Ring AM, Krieg C, Lin JT, Su L, et al. Exploiting a natural conformational switch to engineer an interleukin-2 ‘superkine. Nature. 2012;484:529–33.
Sun ZC, Ren ZH, Yang KT, Liu ZD, Cao SS, Deng SS, et al. A next-generation tumor-targeting IL-2 preferentially promotes tumor-infiltrating CD8+ T-cell response and effective tumor control. Nat Commun. 2019;10:3874.
Létourneau S, van Leeuwen EMM, Krieg C, Martin C, Pantaleo G, Sprent J, et al. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor α subunit CD25. Proc Natl Acad Sci USA. 2010;107:2171–6.
Duysak T, Kim K, Yun M, Jeong JH, Choy HE. Enhanced anti-cancer efficacy of arginine deaminase expressed by tumor-seeking Salmonella Gallinarum. Oncogene. 2024;43:3378–87.
Kim K, Jeong JH, Lim D, Hong Y, Yun M, Min JJ, et al. A novel balanced-lethal host-vector system based on glmS. Plos One. 2013;8:e60511.
Popoff MY, Le Minor L. Antigenic formulas of the Salmonella serovars. 8th ed. Paris: WHO Collaborating Centre for Reference and Research on Salmonella, Institut Pasteur; 2001.
Rhee SH, Im E, Pothoulakis C. Toll-like receptor 5 engagement modulates tumor development and growth in a mouse xenograft model of human colon cancer. Gastroenterology. 2008;135:518–28.
Menendez D, Shatz M, Azzam K, Garantziotis S, Fessler MB, Resnick MA. The Toll-like receptor gene family is integrated into human DNA damage and p53 networks. PLoS Genet. 2011;7:e1001360.
Flentie K, Gonzalez C, Kocher B, Wang Y, Zhu H, Marasa J, et al. Nucleoside diphosphate kinase-3 (NME3) enhances TLR5-induced NFkappaB activation. Mol Cancer Res. 2018;16:986–99.
Lim D, Kim K, Duysak T, So E, Jeong J-H, Choy HE. Bacterial cancer therapy using the attenuated fowl-adapted Salmonella enterica serovar Gallinarum. Mol Therapy Oncolytics. 2023;31:100745.
Tanabe Y, Wada T, Ono K, Abo T, Kutsukake K. The transcript from the σ28-dependent promoter is translationally inert in the expression of the σ28-encoding gene fliA in the fliAZ operon of Salmonella enterica serovar Typhimurium. J Bacteriol. 2011;193:6132–41.
Ide N, Ikebe T, Kutsukake K. Reevaluation of the promoter structure of the class 3 flagellar operons of Escherichia coli and Salmonella. Genes Genet Syst. 1999;74:113–6.
De Boer HA, Comstock LJ, Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci. 1983;80:21–5.
Hopp TP, Prickett KS, Price VL, Libby RT, March CJ, Pat Cerretti D, et al. A short polypeptide marker sequence useful for recombinant protein identification and purification. Bio Technol. 1988;6:1204–10.
Diepold A, Armitage JP. Type III secretion systems: the bacterial flagellum and the injectisome. Philos T R Soc B. 2015;370:20150020.
Hu Z, Zhang T, Jiang S, Yin H. Protocol for evaluation and validation of TLR8 antagonists in HEK-Blue cells via secreted embryonic alkaline phosphatase assay. STAR Protoc. 2022;3:101061.
Ho SN, Abraham RT, Gillis S, McKean DJ. Differential bioassay of interleukin 2 and interleukin 4. J Immunological methods. 1987;98:99–104.
Mai P-T, Lim D, So E, Kim HY, Duysak T, Tran T-Q, et al. Constitutive expression of a cytotoxic anticancer protein in tumor-colonizing bacteria. Cancers. 2023;15:1486.
Vazquez-Lombardi R, Loetsch C, Zinkl D, Jackson J, Schofield P, Deenick EK, et al. Potent antitumour activity of interleukin-2-Fc fusion proteins requires Fc-mediated depletion of regulatory T-cells. Nat Commun. 2017;8:15373.
Zheng JH, Nguyen VH, Jiang SN, Park SH, Tan W, Hong SH, et al. Two-step enhanced cancer immunotherapy with engineered Salmonella typhimurium secreting heterologous flagellin. Sci Transl Med. 2017;9:eaak9537.
Cho D, Campana D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med. 2009;29:89–96.
Wrangle JM, Patterson A, Johnson CB, Neitzke DJ, Mehrotra S, Denlinger CE, et al. IL-2 and beyond in cancer immunotherapy. J Inter Cytok Res. 2018;38:45–68.
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–5.
Kim JE, Phan TX, Nguyen VH, Dinh-Vu HV, Zheng JH, Yun M, et al. Suppresses tumor growth via the pro-inflammatory cytokine interleukin-1β. Theranostics. 2015;5:1328–42.
Jayasingam SD, Citartan M, Thang TH, Zin AAM, Ang KC, Ch’ng ES. Evaluating the polarization of tumor-associated macrophages Into M1 and M2 phenotypes in human cancer tissue: technicalities and challenges in routine clinical practice. Front Oncol. 2020;9:1512.
Gram AM, Wright JA, Pickering RJ, Lam NL, Booty LM, Webster SJ, et al. Flagellin activates NAIP/NLRC4 and canonical NLRP3 inflammasomes in human macrophages. J Immunol. 2021;206:631–40.
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20:651–68.
Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–7.
Bentebibel SE, Hurwitz ME, Bernatchez C, Haymaker C, Hudgens CW, Kluger HM, et al. A first-in-human study and biomarker analysis of NKTR-214, a novel 1L2Rβγ-biased cytokine, in patients with advanced or metastatic solid tumors. Cancer Discov. 2019;9:711–21.
D’Alise AM, Brasu N, De Intinis C, Leoni G, Russo V, Langone F, et al. Adenoviral-based vaccine promotes neoantigen-specific CD8(+) T cell stemness and tumor rejection. Sci Transl Med. 2022;14:eabo7604.
Roser LA, Sommer C, Iannazzo SO, Sakellariou C, Waibler Z, Gogesch P. Revival of recombinant IL-2 therapy - approaches from the past until today. J Immunotoxicol. 2024;21:S38–S47.
Danielli R, Patuzzo R, Di Giacomo AM, Gallino G, Maurichi A, Di Florio A, et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: results of a phase II study. Cancer Immunol Immun. 2015;64:999–1009.
Van Limbergen EJ, Hoeben A, Lieverse RIY, Houben R, Overhof C, Postma A, et al. Toxicity of L19-Interleukin 2 combined with stereotactic body radiation therapy: a phase 1 study. Int J Radiat Oncol. 2021;109:1421–30.
Quixabeira DCA, Zafar S, Santos JM, Cervera-Carrascon V, Havunen R, Kudling TV, et al. Oncolytic adenovirus coding for a variant interleukin 2 (vIL-2) cytokine re-programs the tumor microenvironment and confers enhanced tumor control. Front Immunol. 2021;12:674400.
Imai H, Saio M, Nonaka K, Suwa T, Umemura N, Ouyang GF, et al. Depletion of CD4+CD25+ regulatory T cells enhances interleukin-2-induced antitumor immunity in a mouse model of colon adenocarcinoma. Cancer Sci. 2007;98:416–23.
Natale P, Brüser T, Driessen AJM. Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane -: Distinct translocases and mechanisms. BBA Biomembranes. 2008;1778:1735–56.
Blocker A, Jouihri N, Larquet E, Gounon P, Ebel F, Parsot C, et al. Structure and composition of the ‘needle complex’, a part of its type III secreton. Mol Microbiol. 2001;39:652–63.
Wang D, Wei XD, Kalvakolanu DV, Guo BF, Zhang L. Perspectives on oncolytic in cancer immunotherapy-a promising strategy. Front Immunol. 2021;12:615930.
Nguyen DH, You SH, Ngo HTT, Van Nguyen K, Tran KV, Chu TH, et al. Reprogramming the tumor immune microenvironment using engineered dual-drug loaded. Nat Commun. 2024;15:6680.
Raeber ME, Sahin D, Boyman O. Interleukin-2-based therapies in cancer. Sci Transl Med. 2022;14:eabo5409.
Lechner MG, Karimi SS, Barry-Holson K, Angell TE, Murphy KA, Church CH, et al. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J Immunother. 2013;36:477–89.
Wu J, Bloch N, Chang AY, Bhavsar R, Wang Q, Crawford A, et al. A PD-1-targeted, receptor-masked IL-2 immunocytokine that engages IL-2Ralpha strengthens T cell-mediated anti-tumor therapies. Cell Rep. Med. 2024;5:101747.
Zhao B, Zhao H, Zhao J. Efficacy of PD-1/PD-L1 blockade monotherapy in clinical trials. Ther Adv Med Oncol. 2020;12:1758835920937612.
Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86.
Overwijk WW, Tagliaferri MA, Zalevsky J. Engineering IL-2 to give new life to T cell immunotherapy. Annu Rev Med. 2021;72:281–311.
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (No. NRF-2020M3A9G3080282).
Author information
Authors and Affiliations
Contributions
Conceptualization, Hyon E. Choy and Jae-Ho Jeong; methodology, Kwangsoo Kim; data curation, Thanh Quang Tran and Taner Duysak; writing—original draft preparation, Giang Chau Dang and Thanh Quang Tran; writing—review and editing, Hyon E. Choy, Jae Ho Cho, and Yoonjoo Choi; funding acquisition, Jae-Ho Jeong. All authors have read and agreed to the published version of the manuscript. All authors read and approved of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tran, T.Q., Duysak, T., Kim, K. et al. Anti-cancer effect of interleukin-2 fused to flagellin expressed by tumor-targeting Salmonella. Oncogene 44, 3449–3460 (2025). https://doi.org/10.1038/s41388-025-03504-y
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03504-y