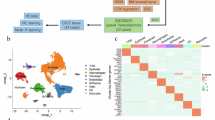
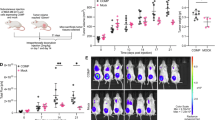
Abstract
Oral squamous cell carcinoma (OSCC) is a highly aggressive malignancy characterized by extensive extracellular matrix (ECM) remodeling and microtubule dynamics, which drive tumor progression and therapeutic resistance. Here, we identify HOMER3 as a novel and pivotal regulator that integrates ECM stiffness and microtubule dynamics to promote OSCC malignancy. HOMER3 expression follows a distinct gradient, increasing from low levels in normal tissues to elevated levels in oral leukoplakia and highest levels in OSCC, with high expression significantly associated with advanced stages and poor survival. Mechanistically, HOMER3 acts as a scaffold protein forming two distinct functional complexes: HOMER3-CAMKK1-TRPV6, which mediates calcium influx and activates AMPK/AKT/mTOR and B-Raf/MEK/ERK pathways to promote proliferation, invasion, and ECM remodeling; and HOMER3-CAMKK1-TUBB3, which regulates microtubule dynamics and drives resistance to the chemotherapeutic agent docetaxel. Functional studies reveal that HOMER3 overexpression enhances ECM stiffness, type I collagen deposition, and Aβ accumulation in the tumor stroma, leading to tumor growth and aggressiveness, while HOMER3 knockdown reduces ECM stiffness, disrupts collagen composition, and increases sensitivity to docetaxel. These findings establish HOMER3 as a pivotal regulator of OSCC malignancy and chemoresistance, providing novel insights into its role in orchestrating the tumor microenvironment and identifying it as a promising therapeutic target for OSCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Data from the research can be accessed from the corresponding author upon reasonable request.
References
Chai AWY, Lim KP, Cheong SC. Translational genomics and recent advances in oral squamous cell carcinoma. Semin Cancer Biol. 2020;61:71–83.
Passirani C, Vessières A, La Regina G, Link W, Silvestri R. Modulating undruggable targets to overcome cancer therapy resistance. Drug Resist Updates. 2022;60:100788.
Zini A, Czerninski R, Sgan-Cohen HD. Oral cancer over four decades: epidemiology, trends, histology, and survival by anatomical sites. J Oral Pathol Med. 2010;39:299–305.
Tan Y, Wang Z, Xu M, Li B, Huang Z, Qin S, et al. Oral squamous cell carcinomas: state of the field and emerging directions. Int J Oral Sci. 2023;15:44.
Chinn SB, Myers JN. Oral cavity carcinoma: current management, controversies, and future directions. J Clin Oncol. 2015;33:3269–76.
Chow LQM. Head and neck cancer. N Engl J Med. 2020;382:60–72.
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6:92.
Chaturvedi P, Singhvi H, Malik A. The role of chronic mucosal trauma in oral cancer: a review of literature. Indian J Med Paediatr Oncol. 2017;38:44.
Lazos JP, Piemonte ED, Lanfranchi HE, Brunotto MN. Characterization of chronic mechanical irritation in oral cancer. Int J Dent. 2017;2017:1–7.
Gupta AA, Kheur S, Varadarajan S, Parveen S, Dewan H, Alhazmi YA, et al. Chronic mechanical irritation and oral squamous cell carcinoma: A systematic review and meta-analysis. Bosn J Basic Med Sci. 2021;21:647–58.
Piemonte ED, Lazos JP, Gilligan GM, Panico RL, Werner LC, Yang YH, et al. Chronic mechanical irritation enhances the effect of tobacco and alcohol on the risk of oral squamous cell carcinoma: a case-control study in Argentina. Clin Oral Investig. 2022;26:6317–26.
Pérez MA, Raimondi AR, Itoiz ME. An experimental model to demonstrate the carcinogenic action of oral chronic traumatic ulcer. J Oral Pathol Med. 2005;34:17–22.
Fujii S, Tajiri Y, Hasegawa K, Matsumoto S, Yoshimoto RU, Wada H, et al. The TRPV4-AKT axis promotes oral squamous cell carcinoma cell proliferation via CaMKII activation. Lab Investig. 2020;100:311–23.
Piccolo S, Panciera T, Contessotto P, Cordenonsi M. YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches. Nat Cancer. 2023;4:9–26.
Kalli M, Poskus MD, Stylianopoulos T, Zervantonakis IK. Beyond matrix stiffness: targeting force-induced cancer drug resistance. Trends Cancer. 2023;9:937–54.
Li C, Qiu S, Liu X, Guo F, Zhai J, Li Z, et al. Extracellular matrix-derived mechanical force governs breast cancer cell stemness and quiescence transition through integrin-DDR signaling. Signal Transduct Target Ther. 2023;8:247.
Wu B, Liu DA, Guan L, Myint PK, Chin L, Dang H, et al. Stiff matrix induces exosome secretion to promote tumour growth. Nat Cell Biol. 2023;25:415–24.
Zhang J, Li J, Hou Y, Lin Y, Zhao H, Shi Y, et al. Osr2 functions as a biomechanical checkpoint to aggravate CD8+ T cell exhaustion in tumor. Cell. 2024;187:3409–26.e24.
Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206.
Ishiguro K, Xavier R. Homer-3 regulates activation of serum response element in T cells via its EVH1 domain. Blood. 2004;103:2248–56.
Sun T, Song C, Zhao G, Feng S, Wei J, Zhang L, et al. HOMER3 promotes non-small cell lung cancer growth and metastasis primarily through GABPB1-mediated mitochondrial metabolism. Cell Death Dis. 2023;14:814.
Liu Q, He L, Li S, Li F, Deng G, Huang X, et al. HOMER3 facilitates growth factor-mediated β-Catenin tyrosine phosphorylation and activation to promote metastasis in triple negative breast cancer. J Hematol Oncol. 2021;14:6.
Chen L, Shan X, Wan X, Zha W, Fan R. HOMER3 promotes liver hepatocellular carcinoma cancer progression by -upregulating EZH2 and mediating miR-361/GPNMB axis. Pathol Res Pract. 2024;254:155150.
Peixoto A, Ferreira D, Azevedo R, Freitas R, Fernandes E, Relvas-Santos M, et al. Glycoproteomics identifies HOMER3 as a potentially targetable biomarker triggered by hypoxia and glucose deprivation in bladder cancer. J Exp Clin Cancer Res. 2021;40:191.
Chen S, Zhang Y, Wang H, Zeng YY, Li Z, Li ML, et al. WW domain-binding protein 2 acts as an oncogene by modulating the activity of the glycolytic enzyme ENO1 in glioma. Cell Death Dis. 2018;9:347.
Tosios KI, Kapranos N, Papanicolaou SI. Loss of basement membrane components laminin and type IV collagen parallels the progression of oral epithelial neoplasia. Histopathology. 1998;33:261–8.
Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801.
Liu Q, Luo Q, Ju Y, Song G. Role of the mechanical microenvironment in cancer development and progression. Cancer Biol Med. 2020;17:282–92.
Bastianello G, Kidiyoor GR, Lowndes C, Li Q, Bonnal R, Godwin J, et al. Mechanical stress during confined migration causes aberrant mitoses and c-MYC amplification. Proc Natl Acad Sci USA. 2024;121:e2404551121.
Mancini A, Gentile MT, Pentimalli F, Cortellino S, Grieco M, Giordano A. Multiple aspects of matrix stiffness in cancer progression. Front Oncol. 2024;14:1406644.
Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–83.
Yoshida GJ. Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways. J Exp Clin Cancer Res. 2020;39:112.
Zhang Z, Wang Z, Liu T, Tang J, Liu Y, Gou T, et al. Exploring the role of ITGB6: fibrosis, cancer, and other diseases. Apoptosis. 2024;29:570–85.
Li W, Chi N, Rathnayake RAC, Wang R. Distinctive roles of fibrillar collagen I and collagen III in mediating fibroblast-matrix interaction: a nanoscopic study. Biochem Biophys Res Commun. 2021;560:66–71.
Lu Z, Li H, Hou C, Peng Y, Long J, Liu J. Endogenously generated amyloid-β increases stiffness in human neuroblastoma cells. Eur Biophys J. 2017;46:415–24.
Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–46.
Ashrafizadeh M, Mirzaei S, Hashemi F, Zarrabi A, Zabolian A, Saleki H, et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed Pharmacother. 2021;141:111824.
Fonteriz RI, De La Fuente S, Moreno A, Lobatón CD, Montero M, Alvarez J. Monitoring mitochondrial [Ca2+] dynamics with rhod-2, ratiometric pericam and aequorin. Cell Calcium. 2010;48:61–69.
Gee KR, Brown KA, Chen WNU, Bishop-Stewart J, Gray D, Johnson I. Chemical and physiological characterization of fluo-4 Ca2+-indicator dyes. Cell Calcium. 2000;27:97–106.
Zhang D, Wang F, Pang Y, Ke X, Zhu S, Zhao E, et al. Down-regulation of CHERP inhibits neuroblastoma cell proliferation and induces apoptosis through ER stress induction. Oncotarget. 2017;8:80956–70.
Keinan N, Pahima H, Ben-Hail D, Shoshan-Barmatz V. The role of calcium in VDAC1 oligomerization and mitochondria-mediated apoptosis. Biochim Biophys Acta. 2013;1833:1745–54.
Rosen L. Membrane depolarization and calcium influx stimulate MEK and MAP kinase via activation of Ras. Neuron. 1994;12:1207–21.
Hwang HY, Shim JS, Kim D, Kwon HJ. Antidepressant drug sertraline modulates AMPK-MTOR signaling-mediated autophagy via targeting mitochondrial VDAC1 protein. Autophagy. 2021;17:2783–99.
Bolanz KA, Hediger MA, Landowski CP. The role of TRPV6 in breast carcinogenesis. Mol Cancer Ther. 2008;7:271–9.
Schmitt JM, Guire ES, Saneyoshi T, Soderling TR. Calmodulin-dependent kinase kinase/calmodulin kinase I activity gates extracellular-regulated kinase-dependent long-term potentiation. J Neurosci. 2005;25:1281–90.
Sun X, Zhang Y, Xin S, Jin L, Cao Q, Wang H, et al. NOTCH3 promotes docetaxel resistance of prostate cancer cells through regulating TUBB3 and MAPK signaling pathway. Cancer Sci. 2024;115:412–26.
Sekino Y, Han X, Kawaguchi T, Babasaki T, Goto K, Inoue S, et al. TUBB3 reverses resistance to docetaxel and cabazitaxel in prostate cancer. Int J Mol Sci. 2019;20:3936.
Thomas P, Mathew D, Anisha K, Ramasubramanian A, Ramalingam K, Ramani P, et al. A retrospective analysis of the clinicopathological profile of oral squamous cell carcinoma in tobacco and non-tobacco users: highlighting the significance of chronic mechanical irritation. Cureus. 2024;16:e59953.
Piemonte E, Lazos J, Belardinelli P, Secchi D, Brunotto M, Lanfranchi-Tizeira H. Oral cancer associated with chronic mechanical irritation of the oral mucosa. Med Oral Patol Oral Cir Bucal. 2018;23:0–0.
Hasegawa K, Fujii S, Matsumoto S, Tajiri Y, Kikuchi A, Kiyoshima T. YAP signaling induces PIEZO to promote oral squamous cell carcinoma cell proliferation. J Pathol. 2021;253:80–93.
Jiang Y, Zhang H, Wang J, Liu Y, Luo T, Hua H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol Oncol. 2022;15:34.
Miura S, Sato K, Kato-Negishi M, Teshima T, Takeuchi S. Fluid shear triggers microvilli formation via mechanosensitive activation of TRPV6. Nat Commun. 2015;6:8871.
Li W, Zhu D, Qin S. SIRT7 suppresses the epithelial-to-mesenchymal transition in oral squamous cell carcinoma metastasis by promoting SMAD4 deacetylation. J Exp Clin Cancer Res. 2018;37:148.
Duran GE, Wang YC, Moisan F, Francisco EB, Sikic BI. Decreased levels of baseline and drug-induced tubulin polymerisation are hallmarks of resistance to taxanes in ovarian cancer cells and are associated with epithelial-to-mesenchymal transition. Br J Cancer. 2017;116:1318–28.
Scherbakov AM, Basharina AA, Sorokin DV, Mikhaevich EI, Mizaeva IE, Mikhaylova AL, et al. Targeting hormone-resistant breast cancer cells with docetaxel: a look inside the resistance. Cancer Drug Resist. 2023;6:103–15.
Berkowitz SA, Wolff J. Intrinsic calcium sensitivity of tubulin polymerization. The contributions of temperature, tubulin concentration, and associated proteins. J Biol Chem. 1981;256:11216–23.
Monteith GR, Prevarskaya N, Roberts-Thomson SJ. The calcium–cancer signalling nexus. Nat Rev Cancer. 2017;17:373–80.
He Y, Khan S, Huo Z, Lv D, Zhang X, Liu X, et al. Proteolysis targeting chimeras (PROTACs) are emerging therapeutics for hematologic malignancies. J Hematol Oncol. 2020;13:103.
Kelm JM, Pandey DS, Malin E, Kansou H, Arora S, Kumar R, et al. PROTAC’ing oncoproteins: targeted protein degradation for cancer therapy. Mol Cancer. 2023;22:62.
Mouillet-Richard S, Martin-Lannerée S, Le Corre D, Hirsch TZ, Ghazi A, Sroussi M, et al. A proof of concept for targeting the PrPC - Amyloid β peptide interaction in basal prostate cancer and mesenchymal colon cancer. Oncogene. 2022;41:4397–404.
Al Khashali H, Ray R, Darweesh B, Wozniak C, Haddad B, Goel S, et al. Amyloid beta leads to decreased acetylcholine levels and non-small cell lung cancer cell survival via a mechanism that involves p38 mitogen-activated protein kinase and protein kinase C in a p53-dependent and -independent manner. Int J Mol Sci. 2024;25:5033.
Tang Y, Zhang D, Robinson S, Zheng J. Inhibition of pancreatic cancer cells by different amyloid proteins reveals an inverse relationship between neurodegenerative diseases and cancer. Adv Biol. 2023;7:e2300070.
Kleffman K, Levinson G, Rose IVL, Blumenberg LM, Shadaloey SAA, Dhabaria A, et al. Melanoma-secreted amyloid beta suppresses neuroinflammation and promotes brain metastasis. Cancer Discov. 2022;12:1314–35.
Acknowledgements
This work was funded by the National Key Research and Development Program of China (2023YFC2506403 to SYS), the Key Program of National Natural Science Foundation of China (82030085 to SYS), the National Natural Science Foundation of China (82202916 to YLY), and the Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZLCX20212300, SSMU-ZLCX20180500). This work was also funded by Shanghai’s Top Priority Research Center (2022ZZ01017).
Author information
Authors and Affiliations
Contributions
SYS and YLY conceived the study and designed the experiments. CHX and JZ conducted the experiments and analyzed data. HZ, LC, JCZ, GZY, RRX, JYZ, and CTF conducted data analysis. All authors analyzed and interpreted the data, contributed to the writing of the manuscript, discussed the results and implications, and edited the manuscript at all stages. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Approval from the Ethics Committee of the Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, was obtained for the human tissue study, and informed consent was provided by all patients involved. All animal experimental procedures were approved by the Animal Care and Use Committee of the Affiliated Ninth People’s Hospital of Shanghai Jiao Tong University School of Medicine.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, C., Zhang, J., Zhang, H. et al. HOMER3 drives oral squamous cell carcinoma progression through TRPV6 calcium influx and TUBB3 microtubule stabilization. Oncogene 45, 278–294 (2026). https://doi.org/10.1038/s41388-025-03611-w
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03611-w