Abstract
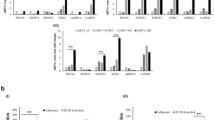
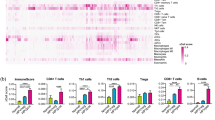
Early intervention of precancers is significant for improving cancer outcome. EZH2-mediated epigenetic modification was responsible for the immune escape of cancers; besides, tumor immune evasion is correlated with the impaired MHC-I antigen presentation machinery (APM). Oral potentially malignant disorders (OPMDs), represented by oral leukoplakia (OLK), usually precede head and neck squamous cell carcinoma (HNSCC). EZH2 is correlated with malignant transformation (MT) of OPMDs including OLK, while it remains undetermined that whether EZH2 mediates the initiation of HNSCC by repressing APM. Herein, EZH2 was first reported to negatively correlate with MHC-I and CD8+ GZMB+ T subsets which promote antitumor immunity in OPMDs. In vitro study uncovered that EZH2 triggers H3K27me3 on the promoters of MHC-I associated genes such as HLA-A/B/C, B2M and TAP1. Next, we constructed one hydrogel loaded with GSK126, a specific EZH2 inhibitor, denoted as PPT@GSK126 which is well-tolerated and highly adhesive to mucosa. Preclinical trials demonstrated that topical PPT@GSK126 could significantly prevent the MT of OPMDs and induce robust specific immune killing of dysplastic cells; while individual local αPD-1 therapy was unavailable, PPT@GSK126 synergized with topical αPD-1 therapy to significantly repress the cancerization of OPMDs. As EZH2 is highly expressed in numerous precancers, PPT@GSK126 has broad application prospects for reducing these tumor burdens.

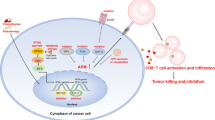
Schematic images presenting the mechanism of action regarding EZH2 in promoting MT of OLK into HNSCC via inhibiting MHC-I associated APM (left panel) and the proposed therapeutic strategy for preventing OLK carcinogenesis (right panel).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive (Genomics, Proteomics & Bioinformatics 2021) in National Genomics Data Center (Nucleic Acids Res 2024), China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA-Human: HRA001006) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa-human.onco.
References
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46.
Tan YS, Sansanaphongpricha K, Prince M, Sun D, Wolf GT, Lei YL. Engineering vaccines to reprogram immunity against head and neck cancer. J Dent Res. 2018;97:627–34.
Liu Y, Liang G, Xu H, Dong W, Dong Z, Qiu Z, et al. Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metab. 2021;33:1221–33.e11.
Ren J, Li N, Pei S, Lian Y, Li L, Peng Y, et al. Histone methyltransferase WHSC1 loss dampens MHC-I antigen presentation pathway to impair IFN-γ-stimulated antitumor immunity. J Clin Investig. 2022;132:e153167.
Xiong J, He J, Zhu J, Pan J, Liao W, Ye H, et al. Lactylation-driven METTL3-mediated RNA m(6)A modification promotes immunosuppression of tumor-infiltrating myeloid cells. Mol Cell. 2022;82:1660–77.e10.
Bais MV. Impact of epigenetic regulation on head and neck squamous cell carcinoma. J Dent Res. 2019;98:268–76.
Li Y, Goldberg EM, Chen X, Xu X, McGuire JT, Leuzzi G, et al. Histone methylation antagonism drives tumor immune evasion in squamous cell carcinomas. Mol Cell. 2022;82:3901–18.e7.
Sun S, Wu Y, Guo W, Yu F, Kong L, Ren Y, et al. STAT3/HOTAIR signaling axis regulates HNSCC growth in an EZH2-dependent manner. Clin Cancer Res. 2018;24:2665–77.
Zhou L, Mudianto T, Ma X, Riley R, Uppaluri R. Targeting EZH2 enhances antigen presentation, antitumor immunity, and circumvents anti-PD-1 resistance in head and neck cancer. Clin Cancer Res. 2020;26:290–300.
Luo X, Qiu Y, Fitzsimonds ZR, Wang Q, Chen Q, Lei YL. Immune escape of head and neck cancer mediated by the impaired MHC-I antigen presentation pathway. Oncogene. 2024;43:388–94.
Vougiouklakis T, Bao R, Nakamura Y, Saloura V. Protein methyltransferases and demethylases dictate CD8+ T-cell exclusion in squamous cell carcinoma of the head and neck. Oncotarget. 2017;8:112797–808.
Johnson DE, Burtness B, Leemans CR, Lui V, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6:92.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67.
Heath BR, Michmerhuizen NL, Donnelly CR, Sansanaphongpricha K, Sun D, Brenner JC, et al. Head and neck cancer immunotherapy beyond the checkpoint blockade. J Dent Res. 2019;98:1073–80.
Rhodus NL. Oral cancer and precancer: improvjing outcomes. Compend Contin Educ Dent. 2009;30:486–8.
Jäwert F, Nyman J, Olsson E, Adok C, Helmersson M, Öhman J. Regular clinical follow-up of oral potentially malignant disorders results in improved survival for patients who develop oral cancer. Oral Oncol. 2021;121:105469.
Aguirre-Urizar JM, Lafuente-Ibáñez de Mendoza I, Warnakulasuriya S. Malignant transformation of oral leukoplakia: systematic review and meta-analysis of the last 5 years. Oral Dis. 2021;27:1881–95.
Warnakulasuriya S, Ariyawardana A. Malignant transformation of oral leukoplakia: a systematic review of observational studies. J Oral Pathol Med. 2016;45:155–66.
Iocca O, Sollecito TP, Alawi F, Weinstein GS, Newman JG, De Virgilio A, et al. Potentially malignant disorders of the oral cavity and oral dysplasia: a systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck. 2020;42:539–55.
Ramos-García P, González-Moles MÁ, Mello FW, Bagan JV, Warnakulasuriya S. Malignant transformation of oral proliferative verrucous leukoplakia: a systematic review and meta-analysis. Oral Dis. 2021;27:1896–907.
Chen XJ, Tan YQ, Zhang N, He MJ, Zhou G. Expression of programmed cell death-ligand 1 in oral squamous cell carcinoma and oral leukoplakia is associated with disease progress and CD8+ tumor-infiltrating lymphocytes. Pathol Res Pract. 2019;215:152418.
Chen Q, Dan H, Pan W, Jiang L, Zhou Y, Luo X, et al. Management of oral leukoplakia: a position paper of the Society of Oral Medicine, Chinese Stomatological Association. Oral Surg Oral Med Oral Pathol Oral Radio. 2021;132:32–43.
Dong Y, Wang Z, Mao F, Cai L, Dan H, Jiang L, et al. PD-1 blockade prevents the progression of oral carcinogenesis. Carcinogenesis. 2021;42:891–902.
Hu S, Lu H, Xie W, Wang D, Shan Z, Xing X, et al. TDO2+ myofibroblasts mediate immune suppression in malignant transformation of squamous cell carcinoma. J Clin Investig. 2022;132:e157649.
Xu SB, Wang MY, Shi XZ, Wang Q, Yu M, Zhang W, et al. Influence of PD-1/PD-L1 on immune microenvironment in oral leukoplakia and oral squamous cell carcinoma. Oral Dis. 2023;29:3268–77.
Lodi G, Franchini R, Warnakulasuriya S, Varoni EM, Sardella A, Kerr AR, et al. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst Rev. 2016;7:CD001829.
Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527:249–53.
Chu W, Zhang X, Qi L, Fu Y, Wang P, Zhao W, et al. The EZH2-PHACTR2-AS1-ribosome axis induces genomic instability and promotes growth and metastasis in breast cancer. Cancer Res. 2020;80:2737–50.
Wang H, Mei Y, Luo C, Huang Q, Wang Z, Lu GM, et al. Single-cell analyses reveal mechanisms of cancer stem cell maintenance and epithelial-mesenchymal transition in recurrent bladder cancer. Clin Cancer Res. 2021;27:6265–78.
Cao W, Younis RH, Li J, Chen H, Xia R, Mao L, et al. EZH2 promotes malignant phenotypes and is a predictor of oral cancer development in patients with oral leukoplakia. Cancer Prev Res (Philos). 2011;4:1816–24.
Ganesh D, Dafar A, Niklasson J, Sandberg I, Braz-Silva P, Sapkota D, et al. EZH2 Expression correlates with T-cell infiltration in oral leukoplakia and predicts cancer transformation. Anticancer Res. 2023;43:1533–42.
Kunju LP, Cookingham C, Toy KA, Chen W, Sabel MS, Kleer CG. EZH2 and ALDH-1 mark breast epithelium at risk for breast cancer development. Mod Pathol. 2011;24:786–93.
Behrens C, Solis LM, Lin H, Yuan P, Tang X, Kadara H, et al. EZH2 protein expression associates with the early pathogenesis, tumor progression, and prognosis of non-small cell lung carcinoma. Clin Cancer Res. 2013;19:6556–65.
Bremer S, Conradi LC, Mechie NC, Amanzada A, Mavropoulou E, Kitz J, et al. Enhancer of zeste homolog 2 in colorectal cancer development and progression. Digestion. 2021;102:227–35.
Huang J, Zhang J, Guo Z, Li C, Tan Z, Wang J, et al. Easy or not-the advances of EZH2 in regulating T cell development, differentiation, and activation in antitumor immunity. Front Immunol. 2021;12:741302.
Morel KL, Sheahan AV, Burkhart DL, Baca SC, Boufaied N, Liu Y, et al. EZH2 inhibition activates a dsRNA-STING-interferon stress axis that potentiates response to PD-1 checkpoint blockade in prostate cancer. Nat Cancer. 2021;2:444–56.
Yap TA, Winter JN, Giulino-Roth L, Longley J, Lopez J, Michot JM, et al. Phase I study of the novel enhancer of zeste homolog 2 (EZH2) inhibitor GSK2816126 in patients with advanced hematologic and solid tumors. Clin Cancer Res. 2019;25:7331–9.
Peng X, Xia X, Xu X, Yang X, Yang B, Zhao P, et al. Ultrafast self-gelling powder mediates robust wet adhesion to promote healing of gastrointestinal perforations. Sci Adv. 2021;7:eabe8739.
Chen B, Zhu D, Li Q, Wang C, Cui J, Zheng Z, et al. Mechanically Reinforced and injectable universal adhesive based on a PEI-PAA/Alg dual-network hydrogel designed by topological entanglement and catechol chemistry. ACS Appl Mater Interfaces. 2023;15:59826–37.
Ni P, Ye S, Xiong S, Zhong M, Shan J, Yuan T, et al. A chitosan-optimized polyethyleneimine/polyacrylic acid multifunctional hydrogel for reducing the risk of ulcerative arterial bleeding. J Mater Chem B. 2023;11:5207–22.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Miranda-Filho A, Bray F. Global patterns and trends in cancers of the lip, tongue and mouth. Oral Oncol. 2020;102:104551.
Webber LP, Wagner VP, Curra M, Vargas PA, Meurer L, Carrard VC, et al. Hypoacetylation of acetyl-histone H3 (H3K9ac) as marker of poor prognosis in oral cancer. Histopathology. 2017;71:278–86.
Mancuso M, Matassa DS, Conte M, Colella G, Rana G, Fucci L, et al. H3K4 histone methylation in oral squamous cell carcinoma. Acta Biochim Pol. 2009;56:405–10.
Burr ML, Sparbier CE, Chan KL, Chan YC, Kersbergen A, Lam E, et al. An evolutionarily conserved function of polycomb silences the MHC class I antigen presentation pathway and enables immune evasion in cancer. Cancer Cell. 2019;36:385–401.e8.
Zingg D, Arenas-Ramirez N, Sahin D, Rosalia RA, Antunes AT, Haeusel J, et al. The histone methyltransferase Ezh2 controls mechanisms of adaptive resistance to tumor immunotherapy. Cell Rep. 2017;20:854–67.
Mahadevan NR, Knelson EH, Wolff JO, Vajdi A, Saigí M, Campisi M, et al. Intrinsic immunogenicity of small cell lung carcinoma revealed by its cellular plasticity. Cancer Discov. 2021;11:1952–69.
Bowen CM, Duzagac F, Martel-Martel A, Reyes-Uribe L, Zaheer M, Thompson J, et al. Inhibition of histone methyltransferase EZH2 for immune interception of colorectal cancer in Lynch syndrome. JCI Insight. 2025;10:e177545.
Guo W, Wang Y, Yang M, Wang Z, Wang Y, Chaurasia S, et al. LincRNA-immunity landscape analysis identifies EPIC1 as a regulator of tumor immune evasion and immunotherapy resistance. Sci Adv. 2021;7:eabb3555.
Edmondson JL, Reed MR, Fil D, Heflin B, McKinnon A, Bauer MA, et al. EZH2 loss during metabolic stress drives restoration of MHC class I machinery in melanoma. iScience. 2025;28:112750.
Stairiker CJ, Thomas GD, Salek-Ardakani S. EZH2 as a regulator of CD8+ T cell fate and function. Front Immunol. 2020;11:593203.
Yu J, Yang K, Zheng J, Zhao P, Xia J, Sun X, et al. Activation of FXR and inhibition of EZH2 synergistically inhibit colorectal cancer through cooperatively accelerating FXR nuclear location and upregulating CDX2 expression. Cell Death Dis. 2022;13:388.
Ding T, Zou J, Qi J, Dan H, Tang F, Zhao H, et al. Mucoadhesive nucleoside-based hydrogel delays oral leukoplakia canceration. J Dent Res. 2022;101:921–30.
Hanna GJ, Villa A, Nandi SP, Shi R, ONeill A, Liu M, et al. Nivolumab for patients with high-risk oral leukoplakia: a nonrandomized controlled trial. JAMA Oncol. 2024;10:32–41.
Chen Q, Wang C, Chen G, Hu Q, Gu Z. Delivery strategies for immune checkpoint blockade. Adv Health Mater. 2018;7:e1800424.
Han X, Li H, Zhou D, Chen Z, Gu Z. Local and targeted delivery of immune checkpoint blockade therapeutics. Acc Chem Res. 2020;53:2521–33.
Acknowledgements
We would like to appreciate all patients participating in this study.
Funding
This work is supported by: National Natural Science Foundation of China 82272899(to XL), 82203180 (to YQ), and 82171809 (to LJ). Research Funding from West China School/Hospital of Stomatology Sichuan University No. RCDWJS2022-16 (to XL). Key Research Program of Sichuan Provincial Science and Technology Agency 2023YFS0127 (to XL). Postdoctoral Research Funding of Sichuan University 2022SCU12132 (to XL). Research and Develop Program, West China Hospital of Stomatology, Sichuan University No. RD-02-202204 (to XL). Youth Fund Projects of Sichuan Provincial Science and Technology Agency 2024NSFSC1904(to YQ). The CAMS Innovation Fund for Medical Sciences CIFMS,2019-I2M-5-004 (to QC). Sichuan Science and Technology Program 2024YFFK0083(to LJ).
Author information
Authors and Affiliations
Contributions
WD, QZ, ZD contributed equally to this work. XL, LJ, and LL contributed equally to this work. Conceptualization: LJ, LL, XL. Methodology: WD, QZ, ZD, QH, HX, JL, CD, YQ. Investigation: WD, YQ, XL, XC, SJ, MH, DP, DY. Visualization: YW, HC, YQ, YJ. Supervision: LJ, LL, XL, LiL, LY, MT, TL, QC, XX. Writing—original draft: WD, QZ, ZD, YQ, XL. Writing—review and editing: XL, LJ, LL, CD.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All experimental procedures were conducted in accordance with the relevant institutional guidelines and regulations. A total of 27 biopsy specimens from patients diagnosed with oral leukoplakia (OLK) and OLK with malignant transformation at the West China Hospital of Stomatology, Sichuan University, were included in this study. Histopathological evaluation of all specimens was independently performed by two certified pathologists. The study protocol was reviewed and approved by the Institutional Review Board of the West China Hospital of Stomatology, Sichuan University (approval numbers: WCHSIRB-AT-2025-402 and WCHSIRB-D-2025-062). Written informed consent was obtained from all participants prior to their inclusion in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, W., Ding, Z., Zeng, Q. et al. Prevention of cancer initiation by augmenting MHC-I antigen presentation via EZH2 inhibition. Oncogene 44, 4878–4894 (2025). https://doi.org/10.1038/s41388-025-03646-z
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03646-z