Abstract
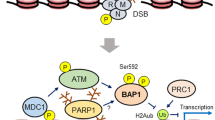
Mesothelioma is an aggressive cancer that is often characterized by loss of the BRCA1-associated protein 1 (BAP1) tumor suppressor gene. This alteration typically occurs as an early clonal event in mesothelioma development, making it a promising candidate for both diagnostic and therapeutic applications. Functionally, BAP1 regulates gene expression through interactions with Polycomb-group complexes, and it plays roles in various other cellular processes including DNA repair, replication stress, and cell metabolism. While preclinical research has identified multiple potential vulnerabilities in BAP1-deficient tumors—including sensitivity to EZH2-, HDAC-, PARP-, and FGFR-inhibitors—translating these findings to the clinic remains a challenge. In this review, we provide a comprehensive overview of BAP1’s molecular functions in mesothelioma, with a focus on their translation into clinical therapeutics for this hard-to-treat malignancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
HM Inspector of Factories and Workshops Annual Report for 1898. London: HMSO; 1899, pp. 171–72.
Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Occup Environ Med. 1960;17:260–71.
Selikoff IJ, Churg J, Hammond EC. Relation between exposure to asbestos and mesothelioma. N Engl J Med. 1965;272:560–5.
Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med. 1992;34:718–21.
Bueno R, Stawiski EW, Goldstein LD, Durinck S, De Rienzo A, Modrusan Z, Gnad F, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–16.
Hmeljak J, Sanchez-Vega F, Hoadley KA, Shih J, Stewart C, Heiman D, Tarpey P, et al. Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov. 2018;8:1548–65.
Bott M, Brevet M, Taylor BS, Shimizu S, Ito T, Wang L, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21. 1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–72.
Testa JR, Cheung M, Pei J, Below JE, Tan Y, Sementino E, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–5.
Walpole S, Pritchard AL, Cebulla CM, Pilarski R, Stautberg M, Davidorf FH, et al. Comprehensive study of the clinical phenotype of germline BAP1 variant-carrying families worldwide. JNCI J Natl Cancer Inst. 2018;110:1328–41.
Carbone M, Minaai M, Kittaneh M, Krausz T, Miettinen MM, Pan Hammarström Q, et al. Clinical and pathologic phenotyping of mesotheliomas developing in carriers of germline BAP1 mutations. J Thorac Oncol. 2025;20:1683–98.
Wu X, Hernandez F-V, Wang H, Wang R, Shiffka S, Shah N, et al. Prospective analysis of mesotheliomas in subjects with BAP1 cancer syndrome: clinical characteristics and epigenetic correlates of disease. J Thorac Oncol. 2025;20:1699–715.
Taylor S, Carpentieri D, Williams J, Acosta J, Southard R. Malignant peritoneal mesothelioma in an adolescent male with BAP1 deletion. J Pediatr Hematol Oncol. 2015;37:e323–e7.
Cebulla CM, Binkley EM, Pilarski R, Massengill JB, Rai K, Liebner DA, et al. Analysis of BAP1 germline gene mutation in young uveal melanoma patients. Ophthalmic Genet. 2015;36:126–31.
White AE, Harper JW. Emerging anatomy of the BAP1 tumor suppressor system. Science. 2012;337:1463–4.
Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, et al. Histone H2A deubiquitinase activity of the polycomb repressive complex PR-DUB. Nature. 2010;465:243–7.
Campagne A, Lee MK, Zielinski D, Michaud A, Le Corre S, Dingli F, et al. BAP1 complex promotes transcription by opposing PRC1-mediated H2A ubiquitylation. Nat Commun. 2019;10:348.
Conway E, Rossi F, Fernandez-Perez D, Ponzo E, Ferrari KJ, Zanotti M, et al. BAP1 enhances Polycomb repression by counteracting widespread H2AK119ub1 deposition and chromatin condensation. Mol cell. 2021;81:3526–.e8.
Fursova NA, Turberfield AH, Blackledge NP, Findlater EL, Lastuvkova A, Huseyin MK, et al. BAP1 constrains pervasive H2AK119ub1 to control the transcriptional potential of the genome. Genes Dev. 2021;35:749–70.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5:210.
Nasu M, Emi M, Pastorino S, Tanji M, Powers A, Luk H, et al. High incidence of somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol. 2015;10:565–76.
Alakus H, Yost SE, Woo B, French R, Lin GY, Jepsen K, et al. BAP1 mutation is a frequent somatic event in peritoneal malignant mesothelioma. J Transl Med. 2015;13:122.
Leblay N, Leprêtre F, Le Stang N, Gautier-Stein A, Villeneuve L, Isaac S, et al. BAP1 is altered by copy number loss, mutation, and/or loss of protein expression in more than 70% of malignant peritoneal mesotheliomas. J Thorac Oncol. 2017;12:724–33.
Zhang M, Luo JL, Sun Q, Harber J, Dawson AG, Nakas A, et al. Clonal architecture in mesothelioma is prognostic and shapes the tumour microenvironment. Nat Commun. 2021;12:1751.
Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, et al. BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 RING finger and enhances BRCA1-mediated cell growth suppression. Oncogene. 1998;16:1097–112.
Kadariya Y, Cheung M, Xu J, Pei J, Sementino E, Menges CW, et al. Bap1 is a bona fide tumor suppressor: genetic evidence from mouse models carrying heterozygous germline Bap1 mutations. Cancer Res. 2016;76:2836–44.
Badhai J, Pandey GK, Song JY, Krijgsman O, Bhaskaran R, Chandrasekaran G, et al. Combined deletion of Bap1, Nf2, and Cdkn2ab causes rapid onset of malignant mesothelioma in mice. J Exp Med. 2020;217:e20191257.
Betti M, Aspesi A, Ferrante D, Sculco M, Righi L, Mirabelli D, et al. Sensitivity to asbestos is increased in patients with mesothelioma and pathogenic germline variants in BAP1 or other DNA repair genes. Genes Chromosomes Cancer. 2018;57:573–83.
Xu J, Kadariya Y, Cheung M, Pei J, Talarchek J, Sementino E, et al. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer Res. 2014;74:4388–97.
Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20:1181–92.
Popat S, Baas P, Faivre-Finn C, Girard N, Nicholson AG, Nowak AK, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up✰. Ann Oncol. 2022;33:129–42.
Cigognetti M, Lonardi S, Fisogni S, Balzarini P, Pellegrini V, Tironi A, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol. 2015;28:1043–57.
Yoshimura M, Kinoshita Y, Hamasaki M, Matsumoto S, Hida T, Oda Y, et al. Highly expressed EZH2 in combination with BAP1 and MTAP loss, as detected by immunohistochemistry, is useful for differentiating malignant pleural mesothelioma from reactive mesothelial hyperplasia. Lung Cancer. 2019;130:187–93.
Cozzi I, Oprescu FA, Rullo E, Ascoli V. Loss of BRCA1-associated protein 1 (BAP 1) expression is useful in diagnostic cytopathology of malignant mesothelioma in effusions. Diagn Cytopathol. 2018;46:9–14.
Andrici J, Sheen A, Sioson L, Wardell K, Clarkson A, Watson N, et al. Loss of expression of BAP1 is a useful adjunct, which strongly supports the diagnosis of mesothelioma in effusion cytology. Mod Pathol. 2015;28:1360–8.
Xie XJ, Liu SY, Chen JY, Zhao Y, Jiang J, Wu L, et al. Development of unenhanced CT-based imaging signature for BAP1 mutation status prediction in malignant pleural mesothelioma: consideration of 2D and 3D segmentation. Lung Cancer. 2021;157:30–39.
Shenouda Mena, Abbas Shaikh Ilana, Deutsch Owen, Mitchell HedyL, Kindler SamuelG, Armato III. Radiomics for differentiation of somatic BAP1 mutation on CT scans of patients with pleural mesothelioma. J Med Imaging. 2024;11:064501.
Baumann F, Flores E, Napolitano A, Kanodia S, Taioli E, Pass H, et al. Mesothelioma patients with germline BAP1 mutations have 7-fold improved long-term survival. Carcinogenesis. 2015;36:76–81.
Pastorino S, Yoshikawa Y, Pass HI, Emi M, Nasu M, Pagano I, et al. A subset of mesotheliomas with improved survival occurring in carriers of BAP1 and other germline mutations. J Clin Oncol. 2018;36:3485–94.
Chua V, Lopez-Anton M, Mizue Terai U, Ryota Tanaka J, Baqai U, Purwin TJ, et al. Slow proliferation of BAP1-deficient uveal melanoma cells is associated with reduced S6 signaling and resistance to nutrient stress. Sci Signal. 2024;17:eadn8376.
Zauderer MG, Bott M, McMillan R, Sima CS, Rusch V, Krug LM, et al. Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J Thorac Oncol. 2013;8:1430–3.
McGregor SM, McElherne J, Minor A, Keller-Ramey J, Dunning R, Husain AN, et al. BAP1 immunohistochemistry has limited prognostic utility as a complement of CDKN2A (p16) fluorescence in situ hybridization in malignant pleural mesothelioma. Hum Pathol. 2017;60:86–94.
Singhi AD, Krasinskas AM, Choudry HA, Bartlett DL, Pingpank JF, Zeh HJ, et al. The prognostic significance of BAP1, NF2, and CDKN2A in malignant peritoneal mesothelioma. Mod Pathol. 2016;29:14–24.
Farzin M, Toon CW, Clarkson A, Sioson L, Watson N, Andrici J, et al. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology. 2015;47:302–7.
Lewis EB. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–70.
Schuettengruber B, Bourbon HM, Di Croce L, Cavalli G. Genome regulation by polycomb and trithorax: 70 years and counting. Cell. 2017;171:34–57.
Blackledge NP, Klose RJ. The molecular principles of gene regulation by polycomb repressive complexes. Nat Rev Mol cell Biol. 2021;22:815–33.
Van Lohuizen M, Verbeek S, Scheljen B, Wientjens E, van der Guidon H, et al. Identification of cooperating oncogenes in Eμ-myc transgenic mice by provirus tagging. Cell. 1991;65:737–52.
Jacobs JJL, Kieboom K, Marino S, DePinho RA, Van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature. 1999;397:164–8.
LaFave LM, Béguelin W, Koche R, Teater M, Spitzer B, Chramiec A, et al. Loss of BAP1 function leads to EZH2-dependent transformation. Nat Med. 2015;21:1344–9.
Zauderer MG, Szlosarek PW, Le Moulec S, Popat S, Taylor P, Planchard D, et al. EZH2 inhibitor tazemetostat in patients with relapsed or refractory, BAP1-inactivated malignant pleural mesothelioma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2022;23:758–67.
Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13:104.
Pinton G, Wang Z, Balzano C, Missaglia S, Tavian D, Boldorini R, et al. CDKN2A determines mesothelioma cell fate to EZH2 inhibition. Front Oncol. 2021;11:678447.
Landman N, Hulsman D, Badhai J, Kopparam J, Puppe J, Pandey GK, et al. Combination of EZH2 and ATM inhibition in BAP1-deficient mesothelioma. Br J Cancer. 2024;130:1855–65.
Badhai J, Landman N, Pandey GK, Song JY, Hulsman D, Krijgsman O, et al. Combined inhibition of EZH2 and FGFR is synergistic in BAP1-deficient malignant mesothelioma. Cancer Res Commun. 2024;4:18–27.
Pandey GK, Landman N, Neikes HK, Hulsman D, Lieftink C, Beijersbergen R, Kolluri KK, et al. Genetic screens reveal new targetable vulnerabilities in BAP1-deficient mesothelioma. Cell Rep Med. 2023;4:100915.
Novelli F, Bononi A, Wang Q, Bai F, Patergnani S, Kricek F, et al. BAP1 forms a trimer with HMGB1 and HDAC1 that modulates gene× environment interaction with asbestos. Proc Natl Acad Sci. 2021;118:e2111946118.
Suarez JS, Novelli F, Goto K, Ehara M, Steele M, Kim JH, et al. HMGB1 released by mesothelial cells drives the development of asbestos-induced mesothelioma. Proc Natl Acad Sci. 2023;120:e2307999120.
Sacco JJ, Kenyani J, Butt Z, Carter R, Chew HY, Cheeseman LP, et al. Loss of the deubiquitylase BAP1 alters class I histone deacetylase expression and sensitivity of mesothelioma cells to HDAC inhibitors. Oncotarget. 2015;6:13757–71.
Kuznetsoff JN, Owens DA, Lopez A, Rodriguez DA, Chee NT, Kurtenbach S, et al. Dual screen for efficacy and toxicity identifies HDAC inhibitor with distinctive activity spectrum for BAP1-mutant uveal melanoma. Mol cancer Res. 2021;19:215–22.
Hurwitz JL, Stasik I, Kerr EM, Holohan C, Redmond KM, McLaughlin KM, et al. Vorinostat/SAHA-induced apoptosis in malignant mesothelioma is FLIP/caspase 8-dependent and HR23B-independent. Eur J Cancer. 2012;48:1096–107.
Krug LM, Kindler HL, Calvert H, Manegold C, Tsao AS, Fennell D, et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol. 2015;16:447–56.
Huang A, Garraway LA, Ashworth A, Weber B. Synthetic lethality as an engine for cancer drug target discovery. Nat Rev Drug Discov. 2020;19:23–38.
Scully R, Chen J, Ochs RL, Keegan K, Hoekstra M, Feunteun J, et al. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell. 1997;90:425–35.
Zhong Q, Chen CF, Li S, Chen Y, Wang CC, Xiao J, et al. Association of BRCA1 with the hRad50-hMre11-p95 complex and the DNA damage response. Science. 1999;285:747–50.
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33.
Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with BRCA1-or BRCA2-mutated breast cancer. N Engl J Med. 2021;384:2394–405.
Lee HS, Seo HR, Lee SA, Choi S, Kang D, Kwon J. BAP1 promotes stalled fork restart and cell survival via INO80 in response to replication stress. Biochem J. 2019;476:3053–66.
Seo HR, Jeong D, Lee S, Lee HS, Lee SA, Kang SW, et al. CHIP and BAP1 act in concert to regulate INO80 ubiquitination and stability for DNA replication. Molecules cells. 2021;44:101–15.
Eletr ZM, Yin L, Wilkinson KD. BAP1 is phosphorylated at serine 592 in S-phase following DNA damage. FEBS Lett. 2013;587:3906–11.
Yu H, Pak H, Hammond-Martel I, Ghram M, Rodrigue A, Daou S, et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc Natl Acad Sci. 2014;111:285–90.
Ismail IH, Davidson R, Gagné JP, Xu ZZ, Poirier GG, Hendzel MJ. Germline mutations in BAP1 impair its function in DNA double-strand break repair. Cancer Res. 2014;74:4282–94.
Singh A, Busacca S, Gaba A, Sheaff M, Poile C, Nakas A, et al. BAP1 loss induces mitotic defects in mesothelioma cells through BRCA1-dependent and independent mechanisms. Oncogene. 2023;42:572–85.
Sato H, Ito T, Hayashi T, Kitano S, Erdjument-Bromage H, Bott MJ, et al. The BAP1 nuclear deubiquitinase is involved in the nonhomologous end-joining pathway of double-strand DNA repair through interaction with DNA-PK. Oncogene. 2024;43:1087–97.
Parrotta R, Okonska A, Ronner M, Weder W, Stahel R, Penengo L, et al. A novel BRCA1-associated protein-1 isoform affects response of mesothelioma cells to drugs impairing BRCA1-mediated DNA repair. J Thorac Oncol. 2017;12:1309–19.
Borchert S, Wessolly M, Schmeller J, Mairinger E, Kollmeier J, Hager T, Mairinger T, Herold T, et al. Gene expression profiling of homologous recombination repair pathway indicates susceptibility for olaparib treatment in malignant pleural mesothelioma in vitro. BMC cancer. 2019;19:108.
Fennell DA, King A, Mohammed S, Branson A, Brookes C, Darlison L, et al. Rucaparib in patients with BAP1-deficient or BRCA1-deficient mesothelioma (MiST1): an open-label, single-arm, phase 2a clinical trial. Lancet Respir Med. 2021;9:593–600.
Passiglia F, Righi L, Bironzo P, Listì A, Farinea G, Capelletto E, et al. Niraparib plus dostarlimab in pleural mesothelioma or non–small cell lung cancer harboring HRR mutations: interim results of the UNITO-001 phase II prospective trial. Clin Cancer Res. 2024;30:959–64.
Ghafoor A, Mian I, Wagner C, Mallory Y, Agra MG, Morrow B, et al. Phase 2 study of olaparib in malignant mesothelioma and correlation of efficacy with germline or somatic mutations in BAP1 gene. JTO Clin Res Rep. 2021;2:100231.
Rathkey D, Khanal M, Murai J, Zhang J, Sengupta M, Jiang Q, et al. Sensitivity of mesothelioma cells to PARP inhibitors is not dependent on BAP1 but is enhanced by temozolomide in cells with high-schlafen 11 and low-O6-methylguanine-DNA methyltransferase expression. J Thorac Oncol. 2020;15:843–59.
Morra F, Merolla F, D’Abbiero D, Ilardi G, Campione S, Monaco R, et al. Analysis of CCDC6 as a novel biomarker for the clinical use of PARP1 inhibitors in malignant pleural mesothelioma. Lung Cancer. 2019;135:56–65.
Fennell D, Hill K, Eminton Z, Griffiths D, Morgan-Fox A, Poile C, et al. Abstract CT263: Efficacy and multiomic analysis of Niraparib in relapsed mesothelioma: NERO a randomized phase II trial. Cancer Res. 2025;85:CT263.
Quispel-Janssen JM, Badhai J, Schunselaar L, Price S, Brammeld J, Iorio F, et al. Comprehensive pharmacogenomic profiling of malignant pleural mesothelioma identifies a subgroup sensitive to FGFR inhibition. Clin Cancer Res. 2018;24:84–94.
Lam WS, Creaney J, Chen FK, Chin WL, Muruganandan S, Arunachalam S, et al. A phase II trial of single oral FGF inhibitor, AZD4547, as second or third line therapy in malignant pleural mesothelioma. Lung Cancer. 2020;140:87–92.
Zauderer MG, Alley EW, Bendell J, Capelletto E, Bauer TM, Callies S, et al. Phase 1 cohort expansion study of LY3023414, a dual PI3K/mTOR inhibitor, in patients with advanced mesothelioma. Invest N Drugs. 2021;39:1081–8.
Drew Y, Zenke FT, Curtin NJ. DNA damage response inhibitors in cancer therapy: lessons from the past, current status and future implications. Nat Rev Drug Discov. 2025;24:19–39.
Bononi A, Yang H, Giorgi C, Patergnani S, Pellegrini L, Su M, Xie G, et al. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ. 2017;24:1694–704.
Baughman JM, Rose CM, Kolumam G, Webster JD, Wilkerson EM, Merrill AE, et al. NeuCode proteomics reveals Bap1 regulation of metabolism. Cell Rep. 2016;16:583–95.
Szlosarek PW, Creelan BC, Sarkodie T, Nolan L, Taylor P, Olevsky O, et al. Pegargiminase plus first-line chemotherapy in patients with nonepithelioid pleural mesothelioma: the ATOMIC-meso randomized clinical trial. JAMA Oncol. 2024;10:475–83.
Barnett SE, Kenyani J, Tripari M, Butt Z, Grosman R, Querques F, et al. BAP1 loss is associated with higher ASS1 expression in epithelioid mesothelioma: implications for therapeutic stratification. Mol Cancer Res. 2023;21:411–27.
Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–86.
Shrestha R, Nabavi N, Lin YY, Mo F, Anderson S, Volik S, et al. BAP1 haploinsufficiency predicts a distinct immunogenic class of malignant peritoneal mesothelioma. Genome Med. 2019;11:8.
Ma X, Lembersky D, Kim ES, Becich MJ, Testa JR, Bruno TC, et al. Spatial landscape of malignant pleural and peritoneal mesothelioma tumor immune microenvironments. Cancer Res Commun. 2024;4:2133–46.
Osmanbeyoglu HU, Palmer D, Sagan A, Sementino E, Becich MJ, Testa JR. Isolated BAP1 genomic alteration in malignant pleural mesothelioma predicts distinct immunogenicity with implications for immunotherapeutic response. Cancers. 2022;14:5626.
Xu D, Gao Y, Yang H, Spils M, Marti TM, Losmanová T, et al. BAP1 deficiency inflames the tumor immune microenvironment and is a candidate biomarker for immunotherapy response in malignant pleural mesothelioma. JTO Clin Res Rep. 2024;5:100672.
Dudnik E, Bar J, Moore A, Gottfried T, Moskovitz M, Dudnik J, et al. BAP1-altered malignant pleural mesothelioma: outcomes with chemotherapy, immune check-point inhibitors and poly (ADP-Ribose) polymerase inhibitors. Front Oncol. 2021;11:603223.
Zhou N, Wu M, Wang C, Yuan M, Cheng Y, Wu H, et al. Malignant mesothelioma with a novel BAP1 germline frameshift mutation treated with dual immune checkpoint inhibitors: a case report. Oncol Lett. 2025;30:1–5.
Reveneau MF, Masliah-Planchon J, Fernandez M, Ouikene A, Dron B, Dadamessi I, et al. Major response of a peritoneal mesothelioma to nivolumab and ipilimumab: a case report, molecular analysis and review of literature. Front Oncol. 2024;14:1410322.
de Gooijer CJ, Baas P, Burgers JA. Current chemotherapy strategies in malignant pleural mesothelioma. Transl Lung Cancer Res. 2018;7:574–83.
Louw A, Panou V, Szejniuk WM, Meristoudis C, Chai SM, van Vliet C, et al. BAP1 loss by immunohistochemistry predicts improved survival to first-line platinum and pemetrexed chemotherapy for patients with pleural mesothelioma: a validation study. J Thorac Oncol. 2022;17:921–30.
Oehl K, Vrugt B, Wagner U, Kirschner MB, Meerang M, Weder W, et al. Alterations in BAP1 are associated with cisplatin resistance through inhibition of apoptosis in malignant pleural mesothelioma. Clin Cancer Res. 2021;27:2277–91.
Kumar N, Alrifai D, Kolluri KK, Sage EK, Ishii Y, Guppy N, et al. Retrospective response analysis of BAP1 expression to predict the clinical activity of systemic cytotoxic chemotherapy in mesothelioma. Lung Cancer. 2019;127:164–6.
Author information
Authors and Affiliations
Contributions
JHLT van Genugten conceived the review, performed the literature search, synthesized the information, and drafted the manuscript. DA Fennell and P Baas provided critical revision of the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
JHLTvG declares no competing interests. DAF reports grants from Aldeyra, Astex Therapeutics, Bayer, BMS and Boehringer Ingelheim, Owkin; non-financial support from BerGenBio, Clovis, Eli Lilly, MSD, Roche, and Tesaro GSK; personal fees from Aldeyra, Cambridge Clinical Laboratories, Ikena, Opna Bio, Owkin, RS Oncology, Roche, MSD. PB reports institutional payments and grants: study grants from BMS, Pfizer, and Roche; consultant and advisory services for MSD, BMS, Aduro, BoehringerIngelheim, Targovax and Verastem.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
van Genugten, J.H., Fennell, D.A. & Baas, P. BAP1-loss in mesothelioma: molecular mechanisms and clinical opportunities. Oncogene 45, 593–602 (2026). https://doi.org/10.1038/s41388-025-03672-x
Received:
Revised:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41388-025-03672-x